
by editor | Jan 29, 2023 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, QAPI, Regulatory Compliance
What is the governing body?
In accordance with the Conditions of Participation, a Medicare certified hospice agency must have a governing body. The governing body has ultimate responsibility for the hospice agency, including legal and financial authority. Medicare Conditions of Participation require that the governing body is informed of the ongoing activities at the hospice agency, including patient care delivery issues and all QAPI activities. The governing body must also appoint a qualified hospice administrator – a hospice employee with the necessary education and experience – who is responsible for hospice daily operations.
The governing body must meet at least quarterly and must maintain written minutes of its meetings.
There are two Conditions of Participation – 418.100 and 418.58 – that relate to the hospice governing body.
Condition of Participation 418.100
This Condition of Participation defines a standard that the governing body is responsible for management of the hospice agency, including its fiscal operations, provision of services, and continuous quality assessment and performance improvement (QAPI) efforts. The governing body also assumes full legal authority of all hospice operations. It further specifies that the governing body should appoint an administrator that reports to the governing body and who is responsible for hospice agency daily operations. The hospice administrator must be a hospice employee and must have necessary training, education, and experience. CMS does not specify the process by which an administrator should be selected by the governing body. If a hospice agency has multiple locations, the governing body is responsible for administration, supervision, and services for all locations as well as for any arranged services.
Condition of Participation 418.58
This Condition of Participation discusses requirements of a hospice agency’s QAPI program. The governing body must ensure that the hospice agency maintains and implements an ongoing quality improvement and patient safety program. Program performance must be monitored on a regular basis. Further, the governing body must ensure that one or more individuals are selected to lead the organization’s QAPI efforts.
The hospice agency’s organization documents must specify that the hospice governing body is responsible for the QAPI program. Additionally, the governing body specifies the frequency of data collection and level of detail of data collected by the QAPI program.
Are there any state regulations?
State hospice licensure regulations may impose additional requirements on the hospice governing body. They may also have specific requirements on the administrator that is selected by the governing body. A hospice is required to meet the most stringent requirements (whether state or federal).
Surveyors will check that all conditions are met. A hospice agency should maintain evidence of the governing body’s role and activities. Governing body authorizations and activities should be documented in governing body meeting minutes, company organization documents, and company policies and procedures.
Where can you find out more?
CMS Conditions of Participation – Governing Body
by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a UPIC?
Unified Program Integrity Contractors (UPICs) are contracted by CMS to conduct detailed medical review, data analysis, and audits of healthcare providers to investigate possibilities of Medicare or Medicaid fraud, waste, and abuse.
While the primary purpose of a RAC or MAC audit is to review payments, the primary purpose of a UPIC audit is to investigate when there is suspicion of fraud – especially fraudulent billing practices. A UPIC audit can lead to federal Medicare fraud charges or criminal prosecution. As such, UPIC audits are more serious than other audits.
What is a UPIC’s scope of responsibility?
Prior to UPICs, Zone Program Integrity Contractors (ZPICs) had been responsible for performing fraud, waste, and abuse detection and prevention activities for CMS. In 2016, CMS began to transition to the UPIC program. This transition took a number of years, with ZPIC contracts rolling over to the UPIC program as ZPIC contracts expired. The ZPIC program has now been phased out and replaced with UPICs. UPICs were formed as part of the Comprehensive Medicaid Integrity Plan (CMIP) with the intention of consolidating under a single federal contractor work performed by numerous Medicare and Medicaid program integrity contractors. UPICs combine all federally funded integrity reviews into a single audit and place payments to all federally funded payers under a higher level of scrutiny.
Consolidating responsibility provides UPICs with access to more data and information about healthcare claims, billing, and payments to hospice agencies. By increasing the level of information and data to which UPICs have access, UPICs have improved ability to identify billing anomalies and fraud.
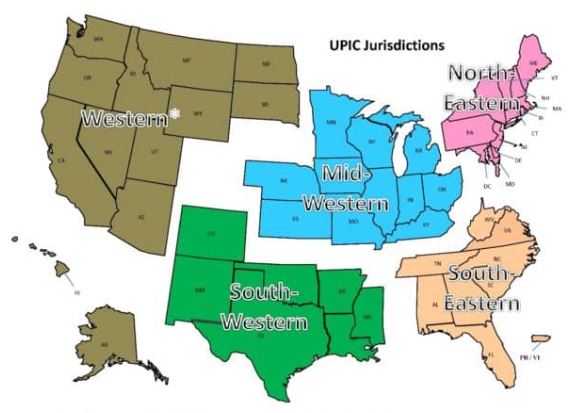
With respect to regional responsibility, the United States has been split into different geographic jurisdictions: Western, Mid-Western, North-Eastern, South-Eastern, and South-Western. Each UPIC is responsible for handling federal-level audits for both Medicare and Medicaid in one of the different geographic jurisdictions.
How is a hospice agency targeted for a UPIC audit?
UPIC audits are usually triggered by statistical analysis of hospice claims and billing data that identifies anomalies in in a hospice agency’s billing. Factors that often lead to a hospice being targeted for a UPIC audit include:
- Billing trends that are inconsistent with industry trends
- Long inpatient stay
- Referral from law enforcement or a federal agency. (For example, a hospice agency may be referred to a UPIC if at the conclusion of a MAC investigation for improper billing, the findings cannot be classified as billing errors or misunderstandings.)
- Complaints to the OIG
- Inaccurate Medicare billing
- Greater frequency of high end services as compared with local or national averages and patterns
What activities may be involved in a UPIC auditor’s investigation?
UPIC audits are focused reviews. A UPIC will request medical records and conduct interviews to determine whether fraud has occurred.. The UPIC’s audit process typically consists of a detailed review of the hospice’s records to confirm all Medicare billings. A UPIC auditor’s activities may be varied and may extend well beyond a review of medical records and documentation including activities such as:
- On site visit
- Interview hospice patients and/or hospice agency employees
- Review clinical, financial, and time production records
- Perform data analysis
- Look for prior agency violations
How does a UPIC audit progress?
A UPIC audit will typically begin with a letter requesting submission of documents – typically within 30 days but sometimes within 15 days. Most UPICs will agree to an extension of time for document submission.
A hospice agency should carefully review the nature of the request. Is the UPIC only requesting administrative and claims related medical records or is the UPIC also requesting documentation relating to the hospice agency’s business practices?
If the UPIC requests information about the hospice agency’s business practices or business relationships – such as its referral sources – this may indicate that the UPIC received information that the hospice agency is engaged in questionable business practices. If the UPIC identifies improper practices, the hospice agency will be referred to the Office of the Inspector General (OIG) or Department of Justice (DOJ).
If the UPIC only reviews claims and the associated medical or billing records, then there are typically two cases:
- Case 1 – The UPIC requests ten or fewer post-payment claims: the UPIC is likely conducting a “Probe Sample”. The purpose of a probe sample is to check if there are problems with the hospice agency’s billing practices, medical necessity, or documentation. This means that the data analyst identified a potentially problematic pattern following the data analysis. The investigator was notified of this pattern and a sample of claims is requested that match the identified pattern. If no significant problems are identified in the initial sample of claims, the UPIC typically issues an “Education Letter”. If numerous problems are found, the UPIC usually expands its audit and issues a request for a larger sample of 30 or more claims.
- The auditor will extrapolate based upon the findings of the 30 or more claims. Extrapolation allows the auditor to identify the error rate in the sample, and then extrapolate the error rate over the entire universe of six years of claims. (Six years is the maximum look back period for claims review.) For example, if the auditor collects a sample of 50 claims and errors are identified in 10 claims, then the error rate is 10/50=20%. It is then assumed that the accuracy of the billing identified in the sample is indicative of the entire universe. Consequently, the error rate of 20% identified in the sample is applied to the entire universe. Even if the hospice agency changed processes, billing software, or billing staff during the duration of time period of the universe, the sample error rate is still applied to the universe. As such, the impact of extrapolation is often quite significant.
- Case 2 – The UPIC requests 30 or more claims: the UPIC likely selected these claims as part of a “Statistically Relevant Sample” and will extrapolate the error rate that it finds to the entire universe of claims.
UPICs also conduct unannounced office visits to hospice agencies. If an office visit occurs, the UPIC will arrive at the office site with written request for patient medical records. They will also interview patients and hospice agency workers.
What may be the outcome of a UPIC audit?
A UPIC audit may result in payment suspension if there are findings that indicate the existence of overpayment, incorrect billing, or fraud.
When a hospice agency is faced with payment suspension, it may follow the standard Medicare appeals process. Legal counsel may be helpful in guiding a hospice agency regarding rights as applied to recoupment and claims withholding.
Payment suspension sometimes occurs without prior notice to the hospice agency. If the agency receives prior notice, it has 15 days to rebut. The UPIC must respond within 15 days of receiving the rebuttal. CMS then determines if the suspension should be removed. In most cases, the suspension remains in place.
Initial payment suspension can last up to 180 days with two unappealable 180 day extension periods.
A hospice may continue to provide services and submit claims while payments are suspended. During the suspension period, payments are not made to the hospice. Instead, payments are made to an escrow account that is managed by the UPIC.
If overpayments are identified, they are taken from the escrow account. The balance remaining in the escrow account is returned to the hospice agency once the audit is completed.
If the UPIC identifies any fraudulent behavior, the activity is referred to the Department of Justice (DOJ) or to the Office of Inspector General (OIG).
What if a hospice agency disagrees with UPIC findings?
A hospice agency may appeal overpayments identified by the UPIC through the Medicare administrative appeals process.
How can a hospice agency prevent UPIC audits?
By increasing their compliance efforts and activities, hospice agencies can prevent UPICs and decrease the chance of a negative outcome from a UPIC audit. More specifically,
- CMS requires that every hospice agency have a compliance team. In addition, compliance reporting duties must be defined.
- A hospice’s compliance plan must be kept current and should include
- How to update coverage guidelines from CMS
- Billing protocols
- Staff hiring and training on protocols
- Documentation guidelines
- HIPAA information and training
- Protocols for cross checking Medicare and Medicaid claim data
- Hospice compliance teams should conduct periodic and random internal audits of patient records, billing documentation, and required signatures. Compliance teams should look out for persistent errors and indications such as is additional biller training is required – either for the team or for a specific biller? Or, is there a new regulation that the team is not familiar with? Is there a physician who is consistently late with signatures? A clinician whose documentation does not look complete or timely? Charts should be audited randomly but on an ongoing basis and indications of the need for self-disclosure should be followed up on. Self-disclosure results in overpayment, but it typically removes a hospice agency from being a target for UPIC audits since it is an indicator that the hospice conducts internal self-audits and returns overpayments, as necessary.
- Hospice agencies can hire third party auditors to conduct chart and coding audits. These third-party auditors can suggest improvements to billing processes or hospice operations to improve compliance with regulations.
- Track all payer document requests and reimbursement denials; these may help identify billing problems before they are identified by an auditor.
Where can you find out more?

by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a TPE?
A Targeted Probe and Educate (TPE) is an audit program that was rolled out by CMS in 2017. The stated goal of this program is to help providers reduce claim denials and appeals. The TPE works to achieve its goal by educating providers in topics that will help to eliminate common mistakes that lead to recoupment of Medicare payments.
Through the TPE program, CMS (through the MAC) works directly with the hospice agency to identify errors and
- Assist or direct in correcting the errors
- Assist to quickly improve when errors are found
- Provide one-on-one help or education
TPE audits are not random spot checks. They are targeted audits. A hospice is identified based upon MAC data analysis or claim review. A hospice with high error rates or unusual billing practices may also be selected for a TPE. TPEs often focus on items with high national error rates. TPEs also focus on items that pose financial risk to Medicare.
TPEs target hospice agencies that fall within these identified risk categories. A hospice agency that is compliant with Medicare policies and billing practices will not be selected for a TPE.
What is the TPE audit process?
The TPE audit process begins with a Notification Letter sent from the MAC to the hospice agency. The Notification Letter explains the TPE program and informs the hospice agency that it has been selected for a TPE audit. It also explains the reason that the agency was included in the TPE and advises that an additional documentation request (ADR) is forthcoming. No response to the Notification Letter is required.
TPE Round 1
The ADR arrives following the Notification Letter. The ADR includes a list of 20-40 claims for which medical records and documentation supporting the claims are requested. This is considered Round 1. The hospice agency must submit the requested documentation.
The MAC reviews the documentation supporting the 20-40 claims to determine if the documentation supports the claims that were submitted.
If the hospice agency is deemed compliant (“no unfavorable findings”) after review of the documents submitted in response to the ADR in round 1,
- Round 1 ends
- No further reviews on that topic for at least one year
If there are unfavorable findings (issues are noted):
- One-on-one educational sessions are offered to the provider
The hospice should participate in the one-on-one education. This education provides the hospice agency with an opportunity to speak directly with the auditor and discuss the errors identified. During these sessions, the MAC guides the hospice agency through error correction. Following the education there is a 45 day period for the hospice to make improvements (e.g., system improvements, process improvements) before another TPE review by the MAC. If the MAC is satisfied that the errors have been corrected, the audit is closed. However, if more errors are identified the hospice agency will be entered into another audit round.
TPE Round 2
A second ADR is sent requesting another 20-40 claims. The hospice agency submits the documentation which is again reviewed by the auditor. The process followed in TPE Round 1 repeats. If there are unfavorable findings, the TPE advances to Round 3.
TPE Round 3
A third ADR is sent requesting another 20-40 claims. If there are still unfavorable findings in Round 3, the hospice agency is referred to CMS for next steps including:
- Shift to 100% prepay review
- Referral to a RAC
- Extrapolation/recoupment
- Other action, as instructed by CMS
What is the MAC looking for in the TPE audit?
During the TPE audit, the MAC is looking for billing mistakes that cause hospice agencies to be non-compliant with regulations. The most common problem identified during a TPE audit is that the documentation does not support terminal prognosis of six months or less.
What if a hospice agency disagrees with the audit findings?
If a hospice agency disagrees with the audit findings, the agency can appeal the results through the Medical Appeals Process. The hospice agency will need to request a redetermination of overpayment by the MAC.
Where can you find out more?

by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a SMRC?
A SMRC is a Supplemental Medical Review Contractor. CMS contracts with SMRCs to conduct medical reviews for Medicare Part A, Medicare Part B, and DME providers. SMRCs are contracted through the Center for Program Integrity/Provider Compliance Group Division of Medical Review and Education (DMRE).
By contracting with Supplemental Medical Review Contractors (SMRCs), CMS helps to lower the Medicare payment rates. In addition, CMS seeks to increase the efficiencies of the medical review function.
What areas does a SMRC focus on?
All services and specialties are subject to review. The focus topics at any point in time are assigned to a SMRC via a formal notification. CMS generates areas of focus from different sources such as: anomalies identified based upon analysis of internal CMS data, federal agencies (e.g., OIG), CERT program, PEPPER reports. Projects and focus areas are typically for a specified time frame.
How does a SMRC conduct its review?
A hospice agency SMRC review begins with an Additional Documentation Request (ADR). The SMRC sends the hospice agency a request for addition documentation for the claims that have been targeted for additional medical review.
The letter from the SMRC specifies which topic area/SMRC project the ADR is linked to and how the claims in the ADR were selected for medical review. The hospice must submit the additional documentation by a specific date, as specified in the letter. A hospice agency can usually request an extension to respond. However, failing to respond is viewed as agreeing with negative findings of the audit and CMS will deem that an overpayment was made and begin recouping funds immediately after the medical records due date specified in the ADR.
Responding to an ADR
It is important to prepare an organized response to the SMRC ADR. Several elements contribute to a good response to the audit:
- Timely response: Timeliness is a critical element. The ADR specifies the due date for response – typically 45 days from the date of the letter. Late response is equivalent to agreeing to negative findings of the audit. Failure to respond timely or to respond at all results in overpayment being deemed and trigger of recoupment.
- Dedicated resources to respond: It is recommended to have specific individuals who are responsible for responding to audits. These would include an oversight team as well as individuals from departments such as compliance and billing. Good internal communication will ensure there is no miscommunication and that all necessary documentation is gathered.
- No missing documentation: Every item that is requested in the ADR must be provided. Missing or incomplete documentation is a top reason for medical review denial and resulting overpayment
- Organization of submitted documents: Documentation should be organized in chronological order so that the submitted documentation presents and organized medical story supporting the claims and billing that was submitted to CMS for payment. That is, the documents submitted should “tell the auditor a story.” They should guide the auditor through the patient’s plan of care and through the patient’s course of therapy.
- Number pages: Number each page that is submitted. This will identify if the auditor is overlooked or is missing any submitted documentation and will facilitate responding to any questions (as questions and responses can refer to the numbered pages). Similarly, it facilitates the calls with the auditors; discussions can refer to numbered pages.
- Include the ADR letter
- Provide a point of contact
- Retain a copy of your submission: Retain a complete copy of everything submitted in response to the ADR.
- Submit the response: Submit your response to the ADR, either by mail, fax, or electronically. Retain proof of submission, including proof of the date (and time, if possible) of submission.
- Contractor portal: this is the preferable method of submission. It is the most efficient method of submitting and it is the fastest way for the contractor to receive the submission
- Fax: Retain a copy of the fax confirmation page, indicating when the fax was sent, confirmation of successful transmission, and number of pages sent
- Mail: Confirm the correct mailing address. Retain proof of mailing.
What happens after the SMRC completes the review?
After the SMRC completes its review of the medical claims, it issues a Review Results Letter to the hospice agency, outlining the findings of the review for each of the claims included in the ADR. The letter also details options available to the hospice should it agree or disagree with SMRC’s findings.
What if the hospice agency agrees with the SMRCs findings?
If the hospice agency agrees with the SMRC’s findings and a finding of overpayment was identified, the hospice follows the standard overpayment process.
What if the hospice agency disagrees with the SMRCs findings?
If the hospice agency does not agree with the SMRC’s findings, it can request a Discussion and Education (D&E) session with the SMRC. During the D&E, the hospice communicates directly with the auditor regarding its medical findings. The hospice may also submit any additional missing documentation. This period also serves as an opportunity for education for the hospice agency about coding and payment policies, to avoid future denials. A D&E must usually be requested within 14 days of the Review Results Letter date. The D&E is then scheduled within 14 days of when it is requested. If, during this session, the hospice agency indicates that it has additional documentation to provide to the SMRC to support the medical review of the claims under review, it has 14 days from the date of the D&E session to submit the documentation. Once the SMRC receives the additional documentation, it will conduct a review within 14 days and then generate a revised final results letter – typically within seven days.
A hospice agency may also decline a D&E session but submit additional missing documentation. In this case, within 14 days of the date of the final results letter the hospice agency must convey its intent to submit additional documentation. The documentation must be received by the SMRC within 30 days from the date of the Review Results Letter. Documentation received later will not be considered.
What is a re-review?
If the hospice agency provides additional documentation in response to the first Review Results Letter, the SMRC will review the additional information provided (re-review) and send a new final review results letter.
How does the SMRC report overpayments?
If the SMRC completes its medical review and finds that improper payments were made to the hospice agency, it will notify the MAC. (Learn more about a MAC here.) The MAC will also be notified if the hospice agency failed to comply with the request to submit documentation. However, the SMRC will wait at least 60 days from the final review letter and 30 days from the re-review before it provides the MAC with the details of the claims that are subject to recoupment. The SMRC compiles the list of claims that are subject to adjustment and sends the details to the MAC. The MAC sends an overpayment demand letter to the hospice agency. Demand letters from the MAC may have appeal rights.
Demand letters and appeal rights
Once an audit is finalized, hospice agencies should look out for the demand letter from the MAC. Questions about overpayments should be directed to the MAC, not to the SMRC. A hospice agency can only appeal once it receives the demand letter.
The agency has 120 days from the MAC demand letter date to file the first level of appeal for redetermination of the SMRC findings. Once the hospice agency files its appeal, collection actions will stop. The MAC will respond to the redetermination within 60 days from receipt of the appeal. The MAC’s response to the appeal for redetermination will include options for additional appeal rights. There are typically options for multiple levels of appeal as well as a final option to elevate the appeal of the claims to an administrative law judge (ALJ). Response time for an appeal to an administrative law judge may be quite lengthy as there has historically been a significant backlog of requests that have been submitted to this level.
What else can help improve a hospice agency’s success in responding to audits?
Hospice agencies often engage outside support to respond to audits, including both legal experts that specialize in healthcare and in responding to audits as well as compliance experts that specialize in hospice, billing, and audit response. These experts can often serve as a highly efficient and effective sources of support and can increase the likelihood of a positive outcome including overturning auditors’ negative findings.
Where can you find more information

by editor | Dec 11, 2022 | Human Resources, Interdisciplinary Team
Hospice agencies rely on the successful coordination of their teams to deliver quality care to their diverse customers and clients who have varying demands and needs. These teams are often highly diverse – with varying cultures, genders, and age ranges.
But simply combining individuals with different backgrounds is not sufficient to ensure customer satisfaction and client success. It also requires inclusive leadership – leaders that create an inclusive environment where team members feel that they belong and matter, where a diverse workforce is respected, and where team members with varying opinions and perspectives feel heard and respected and are willing to share, contribute, and collaborate.
What are the benefits of inclusive leadership?
Workplaces with inclusive leadership are not only nice to have; they are also shown to improve performance. Teams with inclusive leaders are 17% more likely to be high performing, 20% more likely to make high quality decisions, and 29% more likely to be more collaborative, as discussed in a study published by Deloitte.
What are the characteristics of inclusive leadership?
There are several traits that are commonly observed in inclusive leaders:
- Commitment: See the value in diversity and inclusion and holds themselves, the team, and the organization accountable to ensure equality is factored into all processes
- Humility: Modest about their capabilities, admit mistakes, and create space for others to contribute
- Aware of bias: Aware of personal blind spots and flaws in the organizational processes, and work hard to ensure meritocracy
- Cultural intelligence: Exceptional leaders stretch their curiosity and learn about customs that are important to different employees. They aim to boost the level comfort to build inclusivity.
- Effective collaboration: Empower others and focus on team cohesion
- Curiosity about others: Be curious and learn about other employees, acknowledge the value that each individual team member brings to the whole
How can leaders exercise these traits?
Leaders need to practice these traits and get regular feedback on their performance. How can they do this and how can they get regular feedback on their performance?
- Establish a diverse personal advisory board – Leaders can create a group of peers with whom they have regular contact and who they know are willing to give them direct and straightforward feedback on day-to-day interpersonal behavior. Members of their advisory board can give them feedback on activities such as whether they are favoring certain groups, whether their language is language is biased, whether they are inclusive during meetings, and the like.
- Leaders can immerse themselves in new/uncomfortable situations that involve diverse stakeholders, providing them with the opportunity to disrupt preconceived ideas.
- They can share learning journeys that involve recognizing and addressing bias. This can be in the context of town hall meetings or 360 assessments with management. These settings allow a leader to role model the importance of humility.
Summary
Diversity is at the heart of every organization. If properly managed, it can enhance the success of an organization but if improperly managed, it can lead to dissatisfied employees and inability of an organization to achieve its goals. A successful leader must promote and encourage diversity in an organization, as this will draw out each individual employee’s talents and lead to improved organization success.
Where can you find out more?
by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
Which measures are included in HQRP?
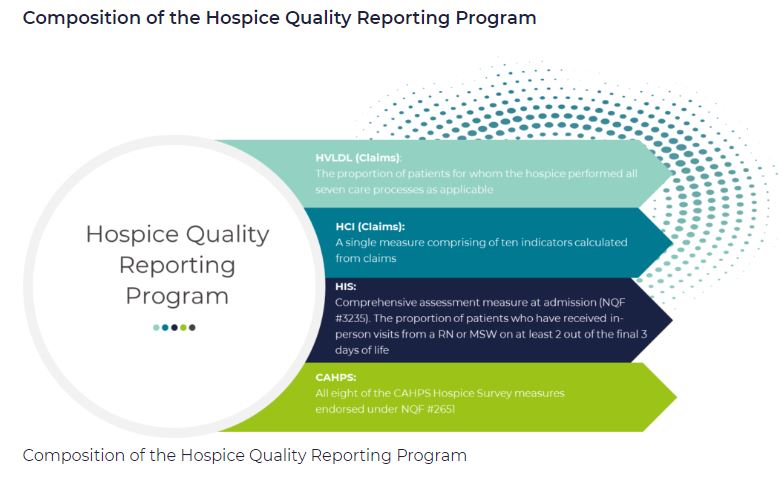
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?






