
by editor | Jan 29, 2023 | Billing, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Intake, Rules and Regulations - Nurses, Rules and Regulations - Office Team
What is the False Claims Act?
The False Claims Act (FCA) was established in 1863 during the Civil War to combat fraud and abuse perpetrated by suppliers of the federal government. At that time, the law was referred to as “Lincoln’s Law.”
The FCA has evolved significantly in recent years and is now one of the main tools used by the government to fight fraud. The FCA penalizes individuals or entities that submit fraudulent claims to the government, cause fraudulent claims to be submitted, or conspire to submit fraudulent claims.
One of the noteworthy provisions of the FCA is the qui tam provision, also known as the whistleblower provision. The qui tam provision allows private citizens, also referred to as “relators”, to report details of alleged fraud to the government. The whistleblower “stands in the shoes” of the government to prosecute the claim. This action benefits the government and the taxpayer as well as potentially the relator, who may receive a share of what is recovered.
How does the FCA relate to a hospice agency?
The False Claims Act allows hospice agency employees, patients, families of patients, or any individuals with alleged knowledge of fraud or abuse by the agency to report the behavior. Under the qui tam provision of the FCA, the relator may be entitled to a percentage of recovered funds.
What are different types of false claims?
A claim is a request for money made to the government. A false claim is money that is obtained from the government due to false or fraudulent claims. False claims include claims where the service
- Has not been provided
- Is already included as part of a different claim (i.e., double billing)
- Is not coded correctly
- Is not supported by the patient’s medical record
Claims may also be false and are covered under the FCA if they result from a referral made in violation of the Federal Anti-kickback statue (Stark Law).
The False Claims Act also includes payment from the government based upon false certification.
False claims include claims that the hospice agency should have known were false or fraudulent.
What is a claim that a hospice agency “should have known” is false?
The FCA expressly includes claims that a hospice agency “should have known” were false or fraudulent. “Should have known” means deliberate ignorance or reckless disregard of truth. As such, a hospice agency cannot avoid liability by simply ignoring inaccuracy in their claims. Examples of “should have known” include:
- Ignorance of billing rules, i.e., lack of knowledge of the rules
- Failure to act on consistent trends that are indicative of inaccurate billing
- Failure to act on inaccuracies or system errors identified by outside or internal auditing teams
- Failure to correct inaccurate billing (impacting either past or future claims)
A hospice agency must understand the rules and take proactive measures — such as conducting internal audits within the organization — to ensure compliance and accurate billing.
How can False Claims Act matters be initiated?
There are two ways that FCA matters can be initiated:
- Initiated by the government: When a FCA matter is initiated by the government, this type of matter typically starts with an audit or an investigation by the government. The government would determine that there is a false claim made to it and would initiate a matter, usually by a subpoena or civil investigative demand (CID). The government would issue the CID directly to the hospice agency. CID is a form of subpoena that requires the hospice agency to engage in one-sided discovery. That is, the hospice agency is required to produce documents demanded, respond to interrogatories, and provide sworn oral testimony. However, the hospice agency may not conduct any discovery.
- Qui tam matter: this type of matter is initiated by a whistleblower, also known as a “relator,” typically through the filing of a sealed lawsuit in a federal district court. The hospice agency does not know about the qui tam lawsuit since the lawsuit is initially served on the government. The case remains under seal while it is investigated by the government.
What is the qui tam process?
Qui tam actions are initially filed under seal. That is, only the US Attorney and some members of the Department of Justice (DOJ) have knowledge of and access to documents related to the case. The relator serves the complaint on the government together with a written disclosure of all material evidence.
The purpose of the sealed qui tam action is to allow the DOJ time to evaluate the relator’s allegations and for the DOJ to decide whether it would like to take over primary responsibility for prosecuting the case. If the DOJ decides to take over primary responsibility for the case, the DOJ is said to “intervene.”
The complaint remains under seal for 60 days during which time the DOJ investigates the relator’s allegations. This 60-day period can be (and typically is) extended. In fact, the government may spend months – or even years – investigating the case.
While the DOJ conducts its investigation, it may issue a Civil Investigative Demand (CID). This form of subpoena requires the defendant (the hospice agency) to engage in one-sided discovery where the hospice agency must produce documents, respond to interrogatories, and provide sworn oral testimony, as demanded. The CID is “one-sided discovery” because the hospice agency may not conduct any discovery.
If the government decides to intervene, the government is then responsible for litigating the case and files its own complaint instead of the complaint that was filed by the relator. The relator remains a party to the complaint.
If the government declines to intervene, the relator may proceed in her own name subject to the government’s right to dismiss the claim or to intervene at a later date.
Whether or not the government decides to intervene, the government remains the real party of interest. (As a reminder, the relator is only “standing in the shoes” of the government.) As such, the government must agree to any decisions on the case. The relator may not agree to dismiss or settle the case without the government’s approval.
What are the key phases in a False Claims Act investigation?
- Phase 1: FCA investigation is triggered. Triggers may include:
- Qui tam (whistleblower) lawsuit
- Call to OIG hotline
- Information identified during audit or claim review
- Complaints
- Data mining
- Phase 2: Formal investigation launches. Investigation may involve:
- Review of corporate filings
- Interview current or former employees
- Review financial records
- Electronic surveillance
- Physical surveillance of employees or of company premises
- DOJ civil investigative demand (CID), or the like
- Government search warrant or raid
- Phase 3: Litigation or resolution
Who are common whistleblowers?
Anyone can be a whistleblower and anyone may report alleged fraudulent activity to the government. The most common relators are:
- Business partners
- Current or former employees
- Competitors
- Patients
- Individuals who mine CMS data to identify anomalies/FCA claims
How can a hospice agency reduce the chance of qui tam lawsuits?
Any complaints or concerns that are raised – by employees, vendors, patients, or competitors, or any other individuals should be investigated and treated with concern as these have the potential to reveal compliance issues that need to be resolved by the hospice agency.
Employee complaints – whether from departing or active employees – are often an excellent source of information on potential compliance issues. A hospice agency should have a clearly established method – that is clearly and often communicated to employees – for employees to raise concerns. It should also have an organized process to diligently investigate and address any concerns raised by employees.
- Internal complaints:
- There must be an organized process – that is communicated regularly to employees – for employees to raise concerns
- All concerns must be investigated
- Have a plan to address any issues that are identified
- Take any necessary corrective actions
- Follow up with the individual who raised the complaint
- Provide training, as needed
- Departing employees
- Treat employees fairly as they leave
- Conduct exit interviews to identify any potential compliance concerns – investigate any issues that may be identified
- Potential releases (e.g., recovery from FCA claims)
Employees must feel that there is a process for raising concerns and that their concerns are heard. Employees should not fear retaliation for raising concerns. A hospice agency should be diligent and careful to respond to all employee complaints that are raised internally or to any complaints that are raised when employees leave the organization.
What are the financial benefits of avoiding FCA violations?
False claims act matters can be quite costly for a hospice organization. In addition to returning the payments associated with the false claims identified and incurring the costs associated with attorney fees to defend the matter, the hospice agency potentially faces the following significant costs:
- Treble damages: The FCA has a treble damages provision which provides that a hospice agency that is found to have violated the FCA statute may be liable to pay three times the amount of the actual false claim amount
- Penalty per claim: Under the FCA, a civil penalty may be assessed for each false claim that is submitted. The civil penalty dollar amount per claim has increased with inflation and currently may be as much as $23,000 per claim.
Where can you find more information?

by editor | Jan 29, 2023 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, QAPI, Regulatory Compliance
What is the governing body?
In accordance with the Conditions of Participation, a Medicare certified hospice agency must have a governing body. The governing body has ultimate responsibility for the hospice agency, including legal and financial authority. Medicare Conditions of Participation require that the governing body is informed of the ongoing activities at the hospice agency, including patient care delivery issues and all QAPI activities. The governing body must also appoint a qualified hospice administrator – a hospice employee with the necessary education and experience – who is responsible for hospice daily operations.
The governing body must meet at least quarterly and must maintain written minutes of its meetings.
There are two Conditions of Participation – 418.100 and 418.58 – that relate to the hospice governing body.
Condition of Participation 418.100
This Condition of Participation defines a standard that the governing body is responsible for management of the hospice agency, including its fiscal operations, provision of services, and continuous quality assessment and performance improvement (QAPI) efforts. The governing body also assumes full legal authority of all hospice operations. It further specifies that the governing body should appoint an administrator that reports to the governing body and who is responsible for hospice agency daily operations. The hospice administrator must be a hospice employee and must have necessary training, education, and experience. CMS does not specify the process by which an administrator should be selected by the governing body. If a hospice agency has multiple locations, the governing body is responsible for administration, supervision, and services for all locations as well as for any arranged services.
Condition of Participation 418.58
This Condition of Participation discusses requirements of a hospice agency’s QAPI program. The governing body must ensure that the hospice agency maintains and implements an ongoing quality improvement and patient safety program. Program performance must be monitored on a regular basis. Further, the governing body must ensure that one or more individuals are selected to lead the organization’s QAPI efforts.
The hospice agency’s organization documents must specify that the hospice governing body is responsible for the QAPI program. Additionally, the governing body specifies the frequency of data collection and level of detail of data collected by the QAPI program.
Are there any state regulations?
State hospice licensure regulations may impose additional requirements on the hospice governing body. They may also have specific requirements on the administrator that is selected by the governing body. A hospice is required to meet the most stringent requirements (whether state or federal).
Surveyors will check that all conditions are met. A hospice agency should maintain evidence of the governing body’s role and activities. Governing body authorizations and activities should be documented in governing body meeting minutes, company organization documents, and company policies and procedures.
Where can you find out more?
CMS Conditions of Participation – Governing Body
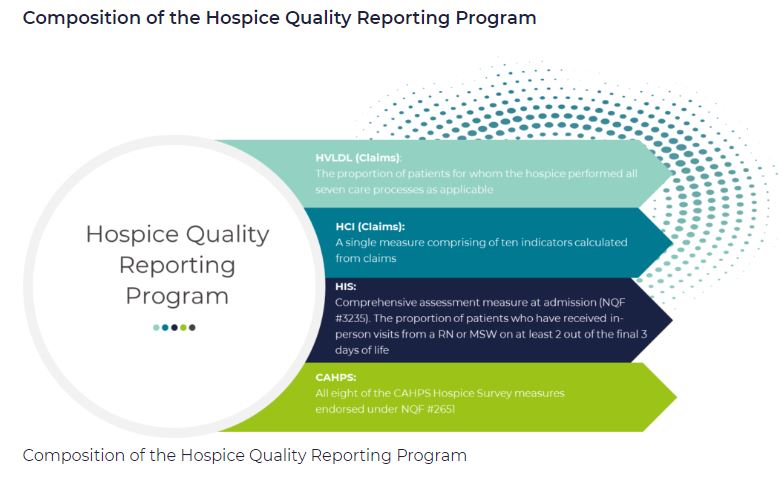
by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
Which measures are included in HQRP?
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
All Medicare certified hospice agencies must submit an HIS Admission and HIS Discharge record on all admissions and discharges from their agency. The report must include all patients, irrespective of payer source, patient age, or location where hospice services were provided. It is recommended that data is submitted within 14 days to be sure that it is accepted within the required 30 day time frame. Submitting early will give the hospice agency time to adjust and correction the data, as needed.
What information is included in each HIS record?
- Admission HIS: Captured during the admission process
- Administrative information
- Preferences
- Active diagnoses
- Health conditions
- Medications
- Record administration
- Discharge HIS: sections of information captured during the discharge process
- Administrative information
- Service utilization (this has been replaced)
- Record administration
How are HIS records used?
The HIS record is used to compute seven process measures:
- Patient treatment preferences
- Beliefs/values address if desired by the patient
- Pain screening
- Pain assessment
- Dyspnea treatment
- Dyspnea screening
- Patients treated with an opioid who are given a bowel regimen
These process measures are combined to compute a single composite quality measure – the Comprehensive Assessment at Admission – that is reported on Care Compare. This composite measure assesses whether the seven key care processes were followed when a patient was admitted to hospice.
What are HIS Submission requirements?
- Within 30 days of patient admission or discharge of each hospice patient. All HIS records must be successfully accepted by QIES ASAP system within 30 calendar days of the patient admission or discharge date. See here for details on submitting HIS data
- – The requirements have included an incrementally increasing compliance threshold since data collection began. The Final Rule stated that beginning with FY 2018 reporting year, to avoid the 2 percentage point reduction in Annual Payment Update (APU), hospice agencies were required to submit at least 70% of their required HIS records within the 30 day deadline. For FY 2019 this minimum threshold was increased to 80% of all required HIS records. For FY 2020 and all subsequent years, the minimum threshold was increased to 90% of all required HIS records within the 30 day deadline. Hospice agencies that meet the submission threshold will avoid the 2% reduction in APU payment.
- – Non compliant providers, that is – providers that fail to meet this submission threshold, receive notification from CMS via a HQRP non-compliance letter that CMS sends via USPS and via the CASPER system. The CASPER letter identifies why the hospice agency is non-compliant and also provides information on how the hospice agency can request reconsideration. Agencies should monitor CASPER for receipt of such notice; agencies have 30 days from the date that the letter is sent for reconsideration.
How can a hospice agency validate that its HIS data has been accepted?
An agency can use reports in CASPER to monitor the status of HIS records submitted to QIES ASAP and track HIS record status, determine when correction of errors is required.
- The Hospice Timeliness Compliance Threshold Report enables a hospice agency to check the timeliness of acceptance of HIS records including the percentage of records that were submitted within the 30 day deadline to determine whether the agency will meet the required threshold.
- The Hospice Final Validation Report provides information on the status of submitted HIS files, indicated whether or not the records were accepted and details of any warning or error messages, if generated.
Where can you get more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
The CAHPS survey is intended to measure the experience of patients who had died while receiving hospice care and the experience of their primary caregivers. It surveys informal caregivers – usually family members – of the persons who died under hospice care.
The survey is a component of the Hospice Quality Reporting Program (HQRP). It is an experience survey rather than a satisfaction survey. The intention of this survey is to provide data that can be publicly reported on Care Compare. It is also intended to provide hospice agencies with data for quality improvement.
How and when is the survey conducted?
To give the caregiver some time for recovery, the survey is administered to the primary informal caregiver of those who died while receiving hospice care at least two months following the month of the patient’s death.
The survey is conducted by mail, by telephone, or by mail with telephone follow up, at the hospice agency’s preference.
How are survey responses reported on Care Compare?
The survey is comprised of 47 questions. Not all of the respondents answer all of the questions and not all of the survey responses are publicly reported. Instead, some of the responses are aggregated together to generate a composite measure that is reported to the public. The result is that eight measures are publicly reported: six composite measures comprised of responses aggregated across multiple questions and two single item measures.
Composite measures:
- Communication with the family
- Receiving timely help
- Treating the patient with respect
- Emotional and spiritual support
- Help for pain and symptoms
- Training the family to care for the patient
Single item measures
- Ratings of the hospice
- Willingness to recommend the hospice
Which hospice agencies must participate in the CAHPS Survey?
Any Medicare certified hospice agency that served at least 50 survey eligible hospice patients in the previous calendar year and that received its CCN after January 1 of the previous calendar year is required to participate in the CAHPS Hospice Survey. The hospice agency is required to successfully submit 12 months of data, from January through December, of the data collection year. Failure to participate will result in a 2% penalty from Medicare payments.
Which agencies are exempt from participating in the survey?
- Newness Exemption: A hospice that receives its CCN on or after January 1 is eligible for a one-time exemption from the CAHPS survey for the remainder of that calendar year. For example, a hospice agency that receives its CCN in 2022 will be required to participate in the CAHPS survey beginning with patients who die in January 2023, unless the agency meets the Size Exemption
- Size Exemption: A hospice agency can apply for an exemption from the CAHPS survey if the agency served fewer than 50 survey eligible patients or caregivers in the prior calendar year. If multiple facilities share a single CCN, the survey eligible patients count is the total from all facilities that share the same CCN. The form to apply for the exemption, submission deadline, and further details on exemption, can be found here
Who administers the CAHPS Hospice Survey?
A hospice agency is not permitted to directly administer the CAHPS hospice survey. Instead, the agency is required to use a CMS approved survey vendor to administer the CAHPS surveys on an ongoing monthly basis.
Where are these results reported?
All eight CAHPS quality measures are publicly reported. They are all also available in the CASPER Preview Reports so that a hospice agency is able to review the data before it is publicly reported on Hospice Care Compare.
May a hospice communicate with its patients and their caregivers about the survey?
If a hospice agency wishes to let its patients know about the CAHPS survey, it must notify all patients about the survey rather than selectively notifying patients. Additionally, the agency cannot try to influence the survey responses or ask caregivers to give certain ratings.
How does CMS adjust the data that is submitted?
The data is case mixed adjusted. That is, CMS tries to remove the effects that arise from the demographics of the patients served by each hospice agency. The intent is to make the scores more comparable across hospice agencies. Data that a hospice agency may receive from its survey vendor may not be case mix adjusted. Consequently, the data that a hospice agency receives from its survey vendor may not match the data that it sees in the CASPER Preview Report or on Hospice Care Compare.
Can a hospice agency review its data before it is publicly reported?
CMS provides a 30 day review period during which providers can use the Hospice CAHPS Provider Preview Report to review their CAHPS data before it is publicly reported on Care Compare. This report can be accessed on CASPER. If a hospice agency finds an error in the data after review the Preview Report, it may request that CMS review the data by submitting a request to the following address hospicecaphssurvey@hsag.com. However, all requests for review must be submitted within 30 days of release of the Preview Report. Detailed instructions for requesting a review of the data can be found here
Where can you find more information?

by editor | Nov 26, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Social Workers
Program for Evaluating Payment Patterns Electronic Reports (PEPPER) is a data analysis report that has been available for hospices since 2012. This report contains claims data statistics based upon UB-04 claims data submissions for a single hospice agency. The statistics are generated for areas that are that are targeted by CMS as at risk for potential of improper Medicare payment. The data is reported in tabular format as well as in graphical format showing a time series of the hospice agency’s performance over time.
What timeframe of data is included in the PEPPER report?
Each PEPPER report summarizes claims data for the most recent three fiscal years (October 1 through September 30).
When is the PEPPER report generated?
The PEPPER report is generated each April. The PEPPER distribution schedule, portal access instructions, and training and resources can be seen here. A PEPPER report is generated for each hospice agency, regardless of whether the agency’s data is concerning or the agency is targeted for additional medical review.
What are PEPPER target areas?
Target areas may change or be added if new areas are identified as target areas for potential risk of improper payment. The report is used by medical reviewers to determine whether a hospice agency should be targeted for additional medical review audits. Hospice PEPPER target areas are the following:
- Live discharges no longer terminally ill: percentage of all hospice patients who were discharged (by death or alive), who were discharged alive excluding patients who were discharged alive due to transfer, revocation, discharge for cause, or patients who moved out of service area
- Live discharges – revocations: percentage of all patients who were discharged (by death or alive) who were discharged alive due to patient revocation
- Live discharges with length of stay between 61-179 days: percentage of all patients who were discharged alive who had a length of stay between 61-179 days
- Long length of stay: percentage of all patients who were discharged (by death or alive) who had a combined length of stay that exceeded 180 days
- Continuous home care provided in assisted living facility: percentage of patients living in assisted living facility for any portion of episode who were discharged (by death or alive) where at least eight hours of continuous care were provided while the patient was in an assisted living facility
- Routing home care provided while patient in assisted living facility: percentage of all routine home care days provided by hospice that were provided while patient was in assisted living facility
- Routing home care provided while patient in nursing facility: percentage of all routine home care days provided by hospice that were provided while patient was in nursing facility
- Routing home care provided while patient in skilled nursing facility: percentage of all routine home care days provided by hospice that were provided while patient was in skilled nursing facility
- Claims with single diagnosis code: percentage of all claims submitted that have a single diagnosis code
- No general inpatient care of continuous home care: percentage of all discharged patients (by death or alive) who had periods of general inpatient care that were longer than five consecutive days
- Average number of Medicare part D claims for beneficiaries residing at home: This metric only includes hospice episodes that are at least three days and that occur at home. For these, the average number of part D claims is computed
- Average number of Medicare part D claims for beneficiaries residing in assisted living facility: This metric only includes hospice episodes that are at least three days and where the patient resides in an assisted living facility. For these, the average number of part D claims is computed
- Average number of Medicare part D claims for beneficiaries residing in a nursing facility: This metric only includes hospice episodes that are at least three days and where the patient resides in a nursing facility. For these, the average number of part D claims is computed
How can a hospice agency use its PEPPER report?
An agency’s PEPPER report contains the agency’s statistics for the target areas as well as comparative statistics: national statistics, state statistics, and MAC jurisdiction statistics. The PEPPER report allows a hospice agency to compare its billing practices to those in its MAC jurisdiction, its state, and across the nation. The PEPPER report also compares the hospice agency’s target values to the national, jurisdictional, and state percentile 80th values for each target area. Agencies that fall above the 80th percentile in any of the target area are considered at risk for improper payment for that risk area. Agencies that score above the 80th percentile in one or multiple target areas will likely be subjects for additional medical review such as TPE, OIG review, or UPIC audit.
- A hospice can use the PEPPER Compare Targets Report to prioritize areas for audit or improvement. The Compare Targets Report reports the hospice agency’s percentile for each target area. A hospice agency can quickly identify if it is at high risk in any target area: if a hospice agency is above the 80th percentile in any target area, the percentile is printed in bold red. Otherwise, all percentiles are printed in black but the agency should check the percentile values. High percentile values (even if below 80) may indicate a potential risk area that the hospice agency should monitor.
- The target area graph shows a bar graph of the hospice agency’s target area value over the three years of data for each of the target areas. The graph also has three trend lines, one for each of the three 80th percentiles for each of the comparison groups. Significant changes in the values over the years should be investigated to be sure that the reason is understood. Is it due to change in staff? Change in management? Change in policies? Or, are there a possibility of improper payments?
- The target area hospice data table contains the various data elements that are used to compute the target area statistic.
- The comparative data table provides the 80th percentile values for the three comparative groups: nation-wide, MAC jurisdiction, and state. The value for any of the comparative groups will be reported as zero if there are fewer than 11 hospices in that group.
How can a hospice agency access its PEPPER report?
PEPPER reports are not sent to hospice agencies. A hospice agency can access its PEPPER report electronically at this website.
Should a hospice access its PEPPER report?
Hospices are encouraged to access their PEPPER reports. The metrics in the PEPPER can support hospice agencies with internal monitoring and auditing. Hospice agencies can use the data to detect trends over time and to compare their performance with that of other agencies – nationally and regionally — to identify outlier behavior and potential anomalies that require further investigation. Reviewing a PEPPER gives a hospice agency the opportunity to improve behaviors or take other corrective actions that can improve the outcome of an audit. It allows a hospice agency to implement self monitoring, self correction, and self improvement which is preferrable to waiting until CMS or a MAC identifies an issue. Additionally, if a hospice is under a large scale audit and has not accessed its PEPPER report, the government may claim that the issues were known or that the agency should have known that it had improper payment practices.
Where can you find more information?






