
by editor | Aug 26, 2024 | Care Keys - Aides, Documentation - Chaplains, Documentation - Nurses, Documentation -Social Workers, QAPI, Uncategorized
What is documentation?
Documentation is an essential part of patient care, especially when you are working as a member of a hospice team. Hospice documentation involves writing down everything you did, observed, and discussed with the patient and their family during your visit. This includes the care you provided, any changes in the patient’s health, and specific events that could impact the patient’s future health. Documentation is like a diary that keeps track of the patient’s journey, making sure everyone involved in the patient’s care is on the same page.
Why is documentation important?
Documentation is a way for the team members to share information about the patient’s health condition and any changes in the patient’s condition. It also serves as legal proof of the care that was provided to the patient. More specifically, there are a number of reasons why documentation is important:
- Communication with the care team: Since hospice care involves multiple healthcare professionals, documentation helps everyone stay informed. Not all members of the care team are present during each patient visit. Notes in the medical record provide vital updates on the patient’s condition and ensure that all members of the care team know how the patient is doing and can make informed decisions about patient care based upon the most up-to-date information about the patient’s health status.
- Legal requirements: Documentation is also a legal requirement. Documentation serves as proof that a patient visit occurred and that specific care was provided. If something goes wrong or if there is a legal question about the patient’s care, the notes in the patient’s medical record can be used to show what happened and when.
- Continuity of care: Proper documentation ensures that the care plan is followed consistently. It helps the team track the patient’s progress and make any changes to the patient’s care, as needed. Without accurate records, important details may be missed which can lead to gaps in the patient’s care.
Guidelines for good documentation
As we have discussed, documentation plays a critical role in helping with communication between the members of the care team, helping meet legal requirements, and in ensuring continuity of patient care. So how can you make sure that the documentation that you write is good documentation — that it is clear, accurate, and useful? The following are some guidelines to follow:
- Document right after the visit: Document your visit immediately after you complete your visit. This helps you remember exactly what happened and ensures that your notes are fresh and accurate.
- Be clear: Think about what you want to write before you start. Make sure your notes are easy for other members of the care team to understand. Write your notes in a way that it is easy to follow, so that others can quickly find the information that they need.
- Stick to the facts: Only write down what actually happened. Avoid including your opinions or assumptions.
- Use quotation marks: If a patient or family member says something important, include their exact words in quotation marks. This helps convey the exact message they intended.
- List your tasks: Write down any tasks you did, helped with, or observed.
- Be specific: Include specific information to help other members of the care team better understand the patient’s current condition. Examples of specific details are (depending on role):
- Patient’s mood
- Any pain or changes in pain
- How well patient moved around
- Changes in appetite
- Weight changes
- Changes in need for oxygen
- Increased or decreased difficulty breathing
Conclusion
Good documentation is more than just writing things down. It is a vital part of providing high quality care. By keeping accurate, detailed, and timely records you help ensure that the patient receives the best possible care and you fulfill your legal responsibilities as a healthcare provider. When in doubt, always ask for help to make sure that your documentation is accurate and useful. Your supervisor, clinical director, or members of your QAPI team are all great resources in case you have questions.
Where can you find more information?

by editor | Jan 29, 2023 | Billing, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Intake, Rules and Regulations - Nurses, Rules and Regulations - Office Team
What is the False Claims Act?
The False Claims Act (FCA) was established in 1863 during the Civil War to combat fraud and abuse perpetrated by suppliers of the federal government. At that time, the law was referred to as “Lincoln’s Law.”
The FCA has evolved significantly in recent years and is now one of the main tools used by the government to fight fraud. The FCA penalizes individuals or entities that submit fraudulent claims to the government, cause fraudulent claims to be submitted, or conspire to submit fraudulent claims.
One of the noteworthy provisions of the FCA is the qui tam provision, also known as the whistleblower provision. The qui tam provision allows private citizens, also referred to as “relators”, to report details of alleged fraud to the government. The whistleblower “stands in the shoes” of the government to prosecute the claim. This action benefits the government and the taxpayer as well as potentially the relator, who may receive a share of what is recovered.
How does the FCA relate to a hospice agency?
The False Claims Act allows hospice agency employees, patients, families of patients, or any individuals with alleged knowledge of fraud or abuse by the agency to report the behavior. Under the qui tam provision of the FCA, the relator may be entitled to a percentage of recovered funds.
What are different types of false claims?
A claim is a request for money made to the government. A false claim is money that is obtained from the government due to false or fraudulent claims. False claims include claims where the service
- Has not been provided
- Is already included as part of a different claim (i.e., double billing)
- Is not coded correctly
- Is not supported by the patient’s medical record
Claims may also be false and are covered under the FCA if they result from a referral made in violation of the Federal Anti-kickback statue (Stark Law).
The False Claims Act also includes payment from the government based upon false certification.
False claims include claims that the hospice agency should have known were false or fraudulent.
What is a claim that a hospice agency “should have known” is false?
The FCA expressly includes claims that a hospice agency “should have known” were false or fraudulent. “Should have known” means deliberate ignorance or reckless disregard of truth. As such, a hospice agency cannot avoid liability by simply ignoring inaccuracy in their claims. Examples of “should have known” include:
- Ignorance of billing rules, i.e., lack of knowledge of the rules
- Failure to act on consistent trends that are indicative of inaccurate billing
- Failure to act on inaccuracies or system errors identified by outside or internal auditing teams
- Failure to correct inaccurate billing (impacting either past or future claims)
A hospice agency must understand the rules and take proactive measures — such as conducting internal audits within the organization — to ensure compliance and accurate billing.
How can False Claims Act matters be initiated?
There are two ways that FCA matters can be initiated:
- Initiated by the government: When a FCA matter is initiated by the government, this type of matter typically starts with an audit or an investigation by the government. The government would determine that there is a false claim made to it and would initiate a matter, usually by a subpoena or civil investigative demand (CID). The government would issue the CID directly to the hospice agency. CID is a form of subpoena that requires the hospice agency to engage in one-sided discovery. That is, the hospice agency is required to produce documents demanded, respond to interrogatories, and provide sworn oral testimony. However, the hospice agency may not conduct any discovery.
- Qui tam matter: this type of matter is initiated by a whistleblower, also known as a “relator,” typically through the filing of a sealed lawsuit in a federal district court. The hospice agency does not know about the qui tam lawsuit since the lawsuit is initially served on the government. The case remains under seal while it is investigated by the government.
What is the qui tam process?
Qui tam actions are initially filed under seal. That is, only the US Attorney and some members of the Department of Justice (DOJ) have knowledge of and access to documents related to the case. The relator serves the complaint on the government together with a written disclosure of all material evidence.
The purpose of the sealed qui tam action is to allow the DOJ time to evaluate the relator’s allegations and for the DOJ to decide whether it would like to take over primary responsibility for prosecuting the case. If the DOJ decides to take over primary responsibility for the case, the DOJ is said to “intervene.”
The complaint remains under seal for 60 days during which time the DOJ investigates the relator’s allegations. This 60-day period can be (and typically is) extended. In fact, the government may spend months – or even years – investigating the case.
While the DOJ conducts its investigation, it may issue a Civil Investigative Demand (CID). This form of subpoena requires the defendant (the hospice agency) to engage in one-sided discovery where the hospice agency must produce documents, respond to interrogatories, and provide sworn oral testimony, as demanded. The CID is “one-sided discovery” because the hospice agency may not conduct any discovery.
If the government decides to intervene, the government is then responsible for litigating the case and files its own complaint instead of the complaint that was filed by the relator. The relator remains a party to the complaint.
If the government declines to intervene, the relator may proceed in her own name subject to the government’s right to dismiss the claim or to intervene at a later date.
Whether or not the government decides to intervene, the government remains the real party of interest. (As a reminder, the relator is only “standing in the shoes” of the government.) As such, the government must agree to any decisions on the case. The relator may not agree to dismiss or settle the case without the government’s approval.
What are the key phases in a False Claims Act investigation?
- Phase 1: FCA investigation is triggered. Triggers may include:
- Qui tam (whistleblower) lawsuit
- Call to OIG hotline
- Information identified during audit or claim review
- Complaints
- Data mining
- Phase 2: Formal investigation launches. Investigation may involve:
- Review of corporate filings
- Interview current or former employees
- Review financial records
- Electronic surveillance
- Physical surveillance of employees or of company premises
- DOJ civil investigative demand (CID), or the like
- Government search warrant or raid
- Phase 3: Litigation or resolution
Who are common whistleblowers?
Anyone can be a whistleblower and anyone may report alleged fraudulent activity to the government. The most common relators are:
- Business partners
- Current or former employees
- Competitors
- Patients
- Individuals who mine CMS data to identify anomalies/FCA claims
How can a hospice agency reduce the chance of qui tam lawsuits?
Any complaints or concerns that are raised – by employees, vendors, patients, or competitors, or any other individuals should be investigated and treated with concern as these have the potential to reveal compliance issues that need to be resolved by the hospice agency.
Employee complaints – whether from departing or active employees – are often an excellent source of information on potential compliance issues. A hospice agency should have a clearly established method – that is clearly and often communicated to employees – for employees to raise concerns. It should also have an organized process to diligently investigate and address any concerns raised by employees.
- Internal complaints:
- There must be an organized process – that is communicated regularly to employees – for employees to raise concerns
- All concerns must be investigated
- Have a plan to address any issues that are identified
- Take any necessary corrective actions
- Follow up with the individual who raised the complaint
- Provide training, as needed
- Departing employees
- Treat employees fairly as they leave
- Conduct exit interviews to identify any potential compliance concerns – investigate any issues that may be identified
- Potential releases (e.g., recovery from FCA claims)
Employees must feel that there is a process for raising concerns and that their concerns are heard. Employees should not fear retaliation for raising concerns. A hospice agency should be diligent and careful to respond to all employee complaints that are raised internally or to any complaints that are raised when employees leave the organization.
What are the financial benefits of avoiding FCA violations?
False claims act matters can be quite costly for a hospice organization. In addition to returning the payments associated with the false claims identified and incurring the costs associated with attorney fees to defend the matter, the hospice agency potentially faces the following significant costs:
- Treble damages: The FCA has a treble damages provision which provides that a hospice agency that is found to have violated the FCA statute may be liable to pay three times the amount of the actual false claim amount
- Penalty per claim: Under the FCA, a civil penalty may be assessed for each false claim that is submitted. The civil penalty dollar amount per claim has increased with inflation and currently may be as much as $23,000 per claim.
Where can you find more information?
by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a UPIC?
Unified Program Integrity Contractors (UPICs) are contracted by CMS to conduct detailed medical review, data analysis, and audits of healthcare providers to investigate possibilities of Medicare or Medicaid fraud, waste, and abuse.
While the primary purpose of a RAC or MAC audit is to review payments, the primary purpose of a UPIC audit is to investigate when there is suspicion of fraud – especially fraudulent billing practices. A UPIC audit can lead to federal Medicare fraud charges or criminal prosecution. As such, UPIC audits are more serious than other audits.
What is a UPIC’s scope of responsibility?
Prior to UPICs, Zone Program Integrity Contractors (ZPICs) had been responsible for performing fraud, waste, and abuse detection and prevention activities for CMS. In 2016, CMS began to transition to the UPIC program. This transition took a number of years, with ZPIC contracts rolling over to the UPIC program as ZPIC contracts expired. The ZPIC program has now been phased out and replaced with UPICs. UPICs were formed as part of the Comprehensive Medicaid Integrity Plan (CMIP) with the intention of consolidating under a single federal contractor work performed by numerous Medicare and Medicaid program integrity contractors. UPICs combine all federally funded integrity reviews into a single audit and place payments to all federally funded payers under a higher level of scrutiny.
Consolidating responsibility provides UPICs with access to more data and information about healthcare claims, billing, and payments to hospice agencies. By increasing the level of information and data to which UPICs have access, UPICs have improved ability to identify billing anomalies and fraud.
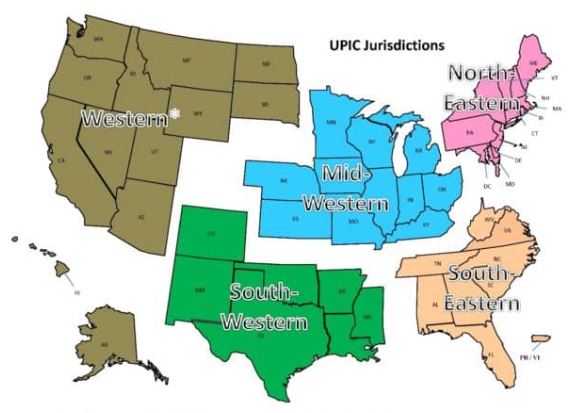
With respect to regional responsibility, the United States has been split into different geographic jurisdictions: Western, Mid-Western, North-Eastern, South-Eastern, and South-Western. Each UPIC is responsible for handling federal-level audits for both Medicare and Medicaid in one of the different geographic jurisdictions.
How is a hospice agency targeted for a UPIC audit?
UPIC audits are usually triggered by statistical analysis of hospice claims and billing data that identifies anomalies in in a hospice agency’s billing. Factors that often lead to a hospice being targeted for a UPIC audit include:
- Billing trends that are inconsistent with industry trends
- Long inpatient stay
- Referral from law enforcement or a federal agency. (For example, a hospice agency may be referred to a UPIC if at the conclusion of a MAC investigation for improper billing, the findings cannot be classified as billing errors or misunderstandings.)
- Complaints to the OIG
- Inaccurate Medicare billing
- Greater frequency of high end services as compared with local or national averages and patterns
What activities may be involved in a UPIC auditor’s investigation?
UPIC audits are focused reviews. A UPIC will request medical records and conduct interviews to determine whether fraud has occurred.. The UPIC’s audit process typically consists of a detailed review of the hospice’s records to confirm all Medicare billings. A UPIC auditor’s activities may be varied and may extend well beyond a review of medical records and documentation including activities such as:
- On site visit
- Interview hospice patients and/or hospice agency employees
- Review clinical, financial, and time production records
- Perform data analysis
- Look for prior agency violations
How does a UPIC audit progress?
A UPIC audit will typically begin with a letter requesting submission of documents – typically within 30 days but sometimes within 15 days. Most UPICs will agree to an extension of time for document submission.
A hospice agency should carefully review the nature of the request. Is the UPIC only requesting administrative and claims related medical records or is the UPIC also requesting documentation relating to the hospice agency’s business practices?
If the UPIC requests information about the hospice agency’s business practices or business relationships – such as its referral sources – this may indicate that the UPIC received information that the hospice agency is engaged in questionable business practices. If the UPIC identifies improper practices, the hospice agency will be referred to the Office of the Inspector General (OIG) or Department of Justice (DOJ).
If the UPIC only reviews claims and the associated medical or billing records, then there are typically two cases:
- Case 1 – The UPIC requests ten or fewer post-payment claims: the UPIC is likely conducting a “Probe Sample”. The purpose of a probe sample is to check if there are problems with the hospice agency’s billing practices, medical necessity, or documentation. This means that the data analyst identified a potentially problematic pattern following the data analysis. The investigator was notified of this pattern and a sample of claims is requested that match the identified pattern. If no significant problems are identified in the initial sample of claims, the UPIC typically issues an “Education Letter”. If numerous problems are found, the UPIC usually expands its audit and issues a request for a larger sample of 30 or more claims.
- The auditor will extrapolate based upon the findings of the 30 or more claims. Extrapolation allows the auditor to identify the error rate in the sample, and then extrapolate the error rate over the entire universe of six years of claims. (Six years is the maximum look back period for claims review.) For example, if the auditor collects a sample of 50 claims and errors are identified in 10 claims, then the error rate is 10/50=20%. It is then assumed that the accuracy of the billing identified in the sample is indicative of the entire universe. Consequently, the error rate of 20% identified in the sample is applied to the entire universe. Even if the hospice agency changed processes, billing software, or billing staff during the duration of time period of the universe, the sample error rate is still applied to the universe. As such, the impact of extrapolation is often quite significant.
- Case 2 – The UPIC requests 30 or more claims: the UPIC likely selected these claims as part of a “Statistically Relevant Sample” and will extrapolate the error rate that it finds to the entire universe of claims.
UPICs also conduct unannounced office visits to hospice agencies. If an office visit occurs, the UPIC will arrive at the office site with written request for patient medical records. They will also interview patients and hospice agency workers.
What may be the outcome of a UPIC audit?
A UPIC audit may result in payment suspension if there are findings that indicate the existence of overpayment, incorrect billing, or fraud.
When a hospice agency is faced with payment suspension, it may follow the standard Medicare appeals process. Legal counsel may be helpful in guiding a hospice agency regarding rights as applied to recoupment and claims withholding.
Payment suspension sometimes occurs without prior notice to the hospice agency. If the agency receives prior notice, it has 15 days to rebut. The UPIC must respond within 15 days of receiving the rebuttal. CMS then determines if the suspension should be removed. In most cases, the suspension remains in place.
Initial payment suspension can last up to 180 days with two unappealable 180 day extension periods.
A hospice may continue to provide services and submit claims while payments are suspended. During the suspension period, payments are not made to the hospice. Instead, payments are made to an escrow account that is managed by the UPIC.
If overpayments are identified, they are taken from the escrow account. The balance remaining in the escrow account is returned to the hospice agency once the audit is completed.
If the UPIC identifies any fraudulent behavior, the activity is referred to the Department of Justice (DOJ) or to the Office of Inspector General (OIG).
What if a hospice agency disagrees with UPIC findings?
A hospice agency may appeal overpayments identified by the UPIC through the Medicare administrative appeals process.
How can a hospice agency prevent UPIC audits?
By increasing their compliance efforts and activities, hospice agencies can prevent UPICs and decrease the chance of a negative outcome from a UPIC audit. More specifically,
- CMS requires that every hospice agency have a compliance team. In addition, compliance reporting duties must be defined.
- A hospice’s compliance plan must be kept current and should include
- How to update coverage guidelines from CMS
- Billing protocols
- Staff hiring and training on protocols
- Documentation guidelines
- HIPAA information and training
- Protocols for cross checking Medicare and Medicaid claim data
- Hospice compliance teams should conduct periodic and random internal audits of patient records, billing documentation, and required signatures. Compliance teams should look out for persistent errors and indications such as is additional biller training is required – either for the team or for a specific biller? Or, is there a new regulation that the team is not familiar with? Is there a physician who is consistently late with signatures? A clinician whose documentation does not look complete or timely? Charts should be audited randomly but on an ongoing basis and indications of the need for self-disclosure should be followed up on. Self-disclosure results in overpayment, but it typically removes a hospice agency from being a target for UPIC audits since it is an indicator that the hospice conducts internal self-audits and returns overpayments, as necessary.
- Hospice agencies can hire third party auditors to conduct chart and coding audits. These third-party auditors can suggest improvements to billing processes or hospice operations to improve compliance with regulations.
- Track all payer document requests and reimbursement denials; these may help identify billing problems before they are identified by an auditor.
Where can you find out more?

by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a TPE?
A Targeted Probe and Educate (TPE) is an audit program that was rolled out by CMS in 2017. The stated goal of this program is to help providers reduce claim denials and appeals. The TPE works to achieve its goal by educating providers in topics that will help to eliminate common mistakes that lead to recoupment of Medicare payments.
Through the TPE program, CMS (through the MAC) works directly with the hospice agency to identify errors and
- Assist or direct in correcting the errors
- Assist to quickly improve when errors are found
- Provide one-on-one help or education
TPE audits are not random spot checks. They are targeted audits. A hospice is identified based upon MAC data analysis or claim review. A hospice with high error rates or unusual billing practices may also be selected for a TPE. TPEs often focus on items with high national error rates. TPEs also focus on items that pose financial risk to Medicare.
TPEs target hospice agencies that fall within these identified risk categories. A hospice agency that is compliant with Medicare policies and billing practices will not be selected for a TPE.
What is the TPE audit process?
The TPE audit process begins with a Notification Letter sent from the MAC to the hospice agency. The Notification Letter explains the TPE program and informs the hospice agency that it has been selected for a TPE audit. It also explains the reason that the agency was included in the TPE and advises that an additional documentation request (ADR) is forthcoming. No response to the Notification Letter is required.
TPE Round 1
The ADR arrives following the Notification Letter. The ADR includes a list of 20-40 claims for which medical records and documentation supporting the claims are requested. This is considered Round 1. The hospice agency must submit the requested documentation.
The MAC reviews the documentation supporting the 20-40 claims to determine if the documentation supports the claims that were submitted.
If the hospice agency is deemed compliant (“no unfavorable findings”) after review of the documents submitted in response to the ADR in round 1,
- Round 1 ends
- No further reviews on that topic for at least one year
If there are unfavorable findings (issues are noted):
- One-on-one educational sessions are offered to the provider
The hospice should participate in the one-on-one education. This education provides the hospice agency with an opportunity to speak directly with the auditor and discuss the errors identified. During these sessions, the MAC guides the hospice agency through error correction. Following the education there is a 45 day period for the hospice to make improvements (e.g., system improvements, process improvements) before another TPE review by the MAC. If the MAC is satisfied that the errors have been corrected, the audit is closed. However, if more errors are identified the hospice agency will be entered into another audit round.
TPE Round 2
A second ADR is sent requesting another 20-40 claims. The hospice agency submits the documentation which is again reviewed by the auditor. The process followed in TPE Round 1 repeats. If there are unfavorable findings, the TPE advances to Round 3.
TPE Round 3
A third ADR is sent requesting another 20-40 claims. If there are still unfavorable findings in Round 3, the hospice agency is referred to CMS for next steps including:
- Shift to 100% prepay review
- Referral to a RAC
- Extrapolation/recoupment
- Other action, as instructed by CMS
What is the MAC looking for in the TPE audit?
During the TPE audit, the MAC is looking for billing mistakes that cause hospice agencies to be non-compliant with regulations. The most common problem identified during a TPE audit is that the documentation does not support terminal prognosis of six months or less.
What if a hospice agency disagrees with the audit findings?
If a hospice agency disagrees with the audit findings, the agency can appeal the results through the Medical Appeals Process. The hospice agency will need to request a redetermination of overpayment by the MAC.
Where can you find out more?

by editor | Jan 28, 2023 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Nurses, Metrics and KPIs, Rules and Regulations - Nurses
What is a SMRC?
A SMRC is a Supplemental Medical Review Contractor. CMS contracts with SMRCs to conduct medical reviews for Medicare Part A, Medicare Part B, and DME providers. SMRCs are contracted through the Center for Program Integrity/Provider Compliance Group Division of Medical Review and Education (DMRE).
By contracting with Supplemental Medical Review Contractors (SMRCs), CMS helps to lower the Medicare payment rates. In addition, CMS seeks to increase the efficiencies of the medical review function.
What areas does a SMRC focus on?
All services and specialties are subject to review. The focus topics at any point in time are assigned to a SMRC via a formal notification. CMS generates areas of focus from different sources such as: anomalies identified based upon analysis of internal CMS data, federal agencies (e.g., OIG), CERT program, PEPPER reports. Projects and focus areas are typically for a specified time frame.
How does a SMRC conduct its review?
A hospice agency SMRC review begins with an Additional Documentation Request (ADR). The SMRC sends the hospice agency a request for addition documentation for the claims that have been targeted for additional medical review.
The letter from the SMRC specifies which topic area/SMRC project the ADR is linked to and how the claims in the ADR were selected for medical review. The hospice must submit the additional documentation by a specific date, as specified in the letter. A hospice agency can usually request an extension to respond. However, failing to respond is viewed as agreeing with negative findings of the audit and CMS will deem that an overpayment was made and begin recouping funds immediately after the medical records due date specified in the ADR.
Responding to an ADR
It is important to prepare an organized response to the SMRC ADR. Several elements contribute to a good response to the audit:
- Timely response: Timeliness is a critical element. The ADR specifies the due date for response – typically 45 days from the date of the letter. Late response is equivalent to agreeing to negative findings of the audit. Failure to respond timely or to respond at all results in overpayment being deemed and trigger of recoupment.
- Dedicated resources to respond: It is recommended to have specific individuals who are responsible for responding to audits. These would include an oversight team as well as individuals from departments such as compliance and billing. Good internal communication will ensure there is no miscommunication and that all necessary documentation is gathered.
- No missing documentation: Every item that is requested in the ADR must be provided. Missing or incomplete documentation is a top reason for medical review denial and resulting overpayment
- Organization of submitted documents: Documentation should be organized in chronological order so that the submitted documentation presents and organized medical story supporting the claims and billing that was submitted to CMS for payment. That is, the documents submitted should “tell the auditor a story.” They should guide the auditor through the patient’s plan of care and through the patient’s course of therapy.
- Number pages: Number each page that is submitted. This will identify if the auditor is overlooked or is missing any submitted documentation and will facilitate responding to any questions (as questions and responses can refer to the numbered pages). Similarly, it facilitates the calls with the auditors; discussions can refer to numbered pages.
- Include the ADR letter
- Provide a point of contact
- Retain a copy of your submission: Retain a complete copy of everything submitted in response to the ADR.
- Submit the response: Submit your response to the ADR, either by mail, fax, or electronically. Retain proof of submission, including proof of the date (and time, if possible) of submission.
- Contractor portal: this is the preferable method of submission. It is the most efficient method of submitting and it is the fastest way for the contractor to receive the submission
- Fax: Retain a copy of the fax confirmation page, indicating when the fax was sent, confirmation of successful transmission, and number of pages sent
- Mail: Confirm the correct mailing address. Retain proof of mailing.
What happens after the SMRC completes the review?
After the SMRC completes its review of the medical claims, it issues a Review Results Letter to the hospice agency, outlining the findings of the review for each of the claims included in the ADR. The letter also details options available to the hospice should it agree or disagree with SMRC’s findings.
What if the hospice agency agrees with the SMRCs findings?
If the hospice agency agrees with the SMRC’s findings and a finding of overpayment was identified, the hospice follows the standard overpayment process.
What if the hospice agency disagrees with the SMRCs findings?
If the hospice agency does not agree with the SMRC’s findings, it can request a Discussion and Education (D&E) session with the SMRC. During the D&E, the hospice communicates directly with the auditor regarding its medical findings. The hospice may also submit any additional missing documentation. This period also serves as an opportunity for education for the hospice agency about coding and payment policies, to avoid future denials. A D&E must usually be requested within 14 days of the Review Results Letter date. The D&E is then scheduled within 14 days of when it is requested. If, during this session, the hospice agency indicates that it has additional documentation to provide to the SMRC to support the medical review of the claims under review, it has 14 days from the date of the D&E session to submit the documentation. Once the SMRC receives the additional documentation, it will conduct a review within 14 days and then generate a revised final results letter – typically within seven days.
A hospice agency may also decline a D&E session but submit additional missing documentation. In this case, within 14 days of the date of the final results letter the hospice agency must convey its intent to submit additional documentation. The documentation must be received by the SMRC within 30 days from the date of the Review Results Letter. Documentation received later will not be considered.
What is a re-review?
If the hospice agency provides additional documentation in response to the first Review Results Letter, the SMRC will review the additional information provided (re-review) and send a new final review results letter.
How does the SMRC report overpayments?
If the SMRC completes its medical review and finds that improper payments were made to the hospice agency, it will notify the MAC. (Learn more about a MAC here.) The MAC will also be notified if the hospice agency failed to comply with the request to submit documentation. However, the SMRC will wait at least 60 days from the final review letter and 30 days from the re-review before it provides the MAC with the details of the claims that are subject to recoupment. The SMRC compiles the list of claims that are subject to adjustment and sends the details to the MAC. The MAC sends an overpayment demand letter to the hospice agency. Demand letters from the MAC may have appeal rights.
Demand letters and appeal rights
Once an audit is finalized, hospice agencies should look out for the demand letter from the MAC. Questions about overpayments should be directed to the MAC, not to the SMRC. A hospice agency can only appeal once it receives the demand letter.
The agency has 120 days from the MAC demand letter date to file the first level of appeal for redetermination of the SMRC findings. Once the hospice agency files its appeal, collection actions will stop. The MAC will respond to the redetermination within 60 days from receipt of the appeal. The MAC’s response to the appeal for redetermination will include options for additional appeal rights. There are typically options for multiple levels of appeal as well as a final option to elevate the appeal of the claims to an administrative law judge (ALJ). Response time for an appeal to an administrative law judge may be quite lengthy as there has historically been a significant backlog of requests that have been submitted to this level.
What else can help improve a hospice agency’s success in responding to audits?
Hospice agencies often engage outside support to respond to audits, including both legal experts that specialize in healthcare and in responding to audits as well as compliance experts that specialize in hospice, billing, and audit response. These experts can often serve as a highly efficient and effective sources of support and can increase the likelihood of a positive outcome including overturning auditors’ negative findings.
Where can you find more information
by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
Which measures are included in HQRP?
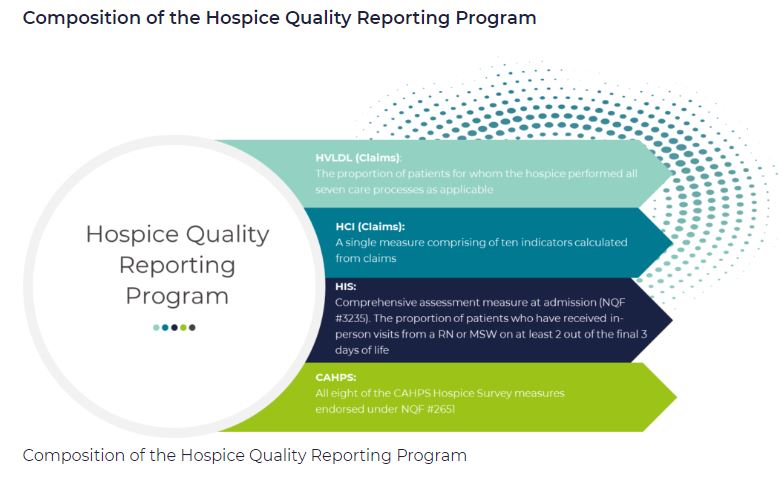
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?






