by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
Which measures are included in HQRP?
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
All Medicare certified hospice agencies must submit an HIS Admission and HIS Discharge record on all admissions and discharges from their agency. The report must include all patients, irrespective of payer source, patient age, or location where hospice services were provided. It is recommended that data is submitted within 14 days to be sure that it is accepted within the required 30 day time frame. Submitting early will give the hospice agency time to adjust and correction the data, as needed.
What information is included in each HIS record?
- Admission HIS: Captured during the admission process
- Administrative information
- Preferences
- Active diagnoses
- Health conditions
- Medications
- Record administration
- Discharge HIS: sections of information captured during the discharge process
- Administrative information
- Service utilization (this has been replaced)
- Record administration
How are HIS records used?
The HIS record is used to compute seven process measures:
- Patient treatment preferences
- Beliefs/values address if desired by the patient
- Pain screening
- Pain assessment
- Dyspnea treatment
- Dyspnea screening
- Patients treated with an opioid who are given a bowel regimen
These process measures are combined to compute a single composite quality measure – the Comprehensive Assessment at Admission – that is reported on Care Compare. This composite measure assesses whether the seven key care processes were followed when a patient was admitted to hospice.
What are HIS Submission requirements?
- Within 30 days of patient admission or discharge of each hospice patient. All HIS records must be successfully accepted by QIES ASAP system within 30 calendar days of the patient admission or discharge date. See here for details on submitting HIS data
- – The requirements have included an incrementally increasing compliance threshold since data collection began. The Final Rule stated that beginning with FY 2018 reporting year, to avoid the 2 percentage point reduction in Annual Payment Update (APU), hospice agencies were required to submit at least 70% of their required HIS records within the 30 day deadline. For FY 2019 this minimum threshold was increased to 80% of all required HIS records. For FY 2020 and all subsequent years, the minimum threshold was increased to 90% of all required HIS records within the 30 day deadline. Hospice agencies that meet the submission threshold will avoid the 2% reduction in APU payment.
- – Non compliant providers, that is – providers that fail to meet this submission threshold, receive notification from CMS via a HQRP non-compliance letter that CMS sends via USPS and via the CASPER system. The CASPER letter identifies why the hospice agency is non-compliant and also provides information on how the hospice agency can request reconsideration. Agencies should monitor CASPER for receipt of such notice; agencies have 30 days from the date that the letter is sent for reconsideration.
How can a hospice agency validate that its HIS data has been accepted?
An agency can use reports in CASPER to monitor the status of HIS records submitted to QIES ASAP and track HIS record status, determine when correction of errors is required.
- The Hospice Timeliness Compliance Threshold Report enables a hospice agency to check the timeliness of acceptance of HIS records including the percentage of records that were submitted within the 30 day deadline to determine whether the agency will meet the required threshold.
- The Hospice Final Validation Report provides information on the status of submitted HIS files, indicated whether or not the records were accepted and details of any warning or error messages, if generated.
Where can you get more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
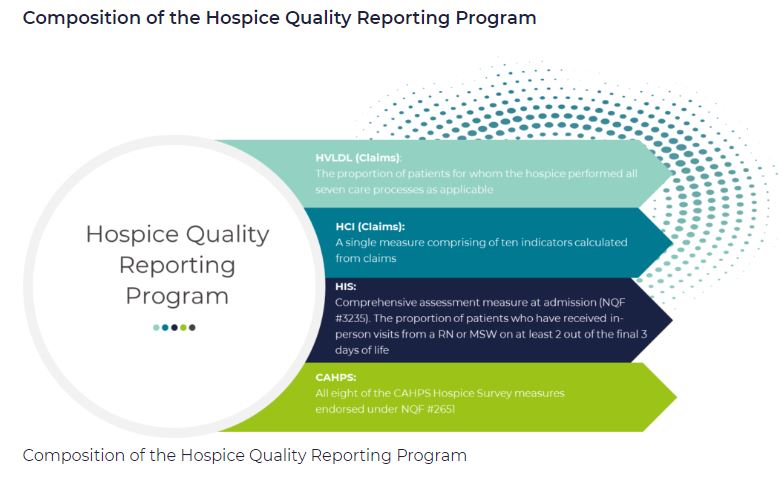
The CAHPS survey is intended to measure the experience of patients who had died while receiving hospice care and the experience of their primary caregivers. It surveys informal caregivers – usually family members – of the persons who died under hospice care.
The survey is a component of the Hospice Quality Reporting Program (HQRP). It is an experience survey rather than a satisfaction survey. The intention of this survey is to provide data that can be publicly reported on Care Compare. It is also intended to provide hospice agencies with data for quality improvement.
How and when is the survey conducted?
To give the caregiver some time for recovery, the survey is administered to the primary informal caregiver of those who died while receiving hospice care at least two months following the month of the patient’s death.
The survey is conducted by mail, by telephone, or by mail with telephone follow up, at the hospice agency’s preference.
How are survey responses reported on Care Compare?
The survey is comprised of 47 questions. Not all of the respondents answer all of the questions and not all of the survey responses are publicly reported. Instead, some of the responses are aggregated together to generate a composite measure that is reported to the public. The result is that eight measures are publicly reported: six composite measures comprised of responses aggregated across multiple questions and two single item measures.
Composite measures:
- Communication with the family
- Receiving timely help
- Treating the patient with respect
- Emotional and spiritual support
- Help for pain and symptoms
- Training the family to care for the patient
Single item measures
- Ratings of the hospice
- Willingness to recommend the hospice
Which hospice agencies must participate in the CAHPS Survey?
Any Medicare certified hospice agency that served at least 50 survey eligible hospice patients in the previous calendar year and that received its CCN after January 1 of the previous calendar year is required to participate in the CAHPS Hospice Survey. The hospice agency is required to successfully submit 12 months of data, from January through December, of the data collection year. Failure to participate will result in a 2% penalty from Medicare payments.
Which agencies are exempt from participating in the survey?
- Newness Exemption: A hospice that receives its CCN on or after January 1 is eligible for a one-time exemption from the CAHPS survey for the remainder of that calendar year. For example, a hospice agency that receives its CCN in 2022 will be required to participate in the CAHPS survey beginning with patients who die in January 2023, unless the agency meets the Size Exemption
- Size Exemption: A hospice agency can apply for an exemption from the CAHPS survey if the agency served fewer than 50 survey eligible patients or caregivers in the prior calendar year. If multiple facilities share a single CCN, the survey eligible patients count is the total from all facilities that share the same CCN. The form to apply for the exemption, submission deadline, and further details on exemption, can be found here
Who administers the CAHPS Hospice Survey?
A hospice agency is not permitted to directly administer the CAHPS hospice survey. Instead, the agency is required to use a CMS approved survey vendor to administer the CAHPS surveys on an ongoing monthly basis.
Where are these results reported?
All eight CAHPS quality measures are publicly reported. They are all also available in the CASPER Preview Reports so that a hospice agency is able to review the data before it is publicly reported on Hospice Care Compare.
May a hospice communicate with its patients and their caregivers about the survey?
If a hospice agency wishes to let its patients know about the CAHPS survey, it must notify all patients about the survey rather than selectively notifying patients. Additionally, the agency cannot try to influence the survey responses or ask caregivers to give certain ratings.
How does CMS adjust the data that is submitted?
The data is case mixed adjusted. That is, CMS tries to remove the effects that arise from the demographics of the patients served by each hospice agency. The intent is to make the scores more comparable across hospice agencies. Data that a hospice agency may receive from its survey vendor may not be case mix adjusted. Consequently, the data that a hospice agency receives from its survey vendor may not match the data that it sees in the CASPER Preview Report or on Hospice Care Compare.
Can a hospice agency review its data before it is publicly reported?
CMS provides a 30 day review period during which providers can use the Hospice CAHPS Provider Preview Report to review their CAHPS data before it is publicly reported on Care Compare. This report can be accessed on CASPER. If a hospice agency finds an error in the data after review the Preview Report, it may request that CMS review the data by submitting a request to the following address [email protected]. However, all requests for review must be submitted within 30 days of release of the Preview Report. Detailed instructions for requesting a review of the data can be found here
Where can you find more information?

by editor | Nov 26, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
The PEPPER report target areas focus on statistics that will identify potential for improper Medicare billing. Comparison to national, jurisdictional, and state percentiles can highlight a hospice agency’s potential need for change to its practices to guard against improper billing. Hospice agencies can leverage the information on these reports to prioritize internal audit and efforts to ensure accurate billing.
Why did CMS focus on the target areas in the PEPPER report?
- Concern: Are patients eligible and is the agency providing good quality of care?
- Target Areas: Live discharges – no longer terminally ill or patient revocation
- Discussion: A hospice may discharge a patient alive because the patient is no longer eligible, the patient revoked, the patient moved out of the service area, or for cause. The first two reasons are concerning. These reasons for live discharge may indicate that the hospice is admitting patients who are not hospice eligible. If the patient revoked it may indicate that the quality of care is lacking.
- Concern: Is a hospice agency trying to take advantage of the high routine home care rate?
- Target Area: Live discharge with length of stay between 61-179 days
- Discussion: CMS pays a higher rate for the first 60 days of routine home care; the rate of payment is lower for days 61+. High incidence of live discharge in days 61+ may indicate the hospice agency is driven by financial concerns and wants to discharge patients once the rate drops.
- Concern: Is the hospice agency admitting ineligible patients?
- Target Area: Long length of stay
- Discussion: The hospice may be admitting ineligible patients and therefore have an unusually long length of stay as compared to its peers
- Concern: Is the hospice agency targeting patients in more profitable care settings?
- Target Area: Services provided in assisted living facilities
- Discussion: An OIG study published in January 2015 found that Medicare payments for hospice care to patients in assisted living facilities increased significantly. While the diagnoses of patients in this setting typically involved less complex care, these patients remained on hospice longer and hospices received higher payments than for patients in other settings. There is therefore a need to monitor whether hospices are targeting patients in more profitable care settings including assisted living facilities, skilled nursing facilities, and nursing facilities.
- Concern: Is a hospice agency accurately reporting all diagnoses on the claim?
- Target Area: Claims with a single diagnosis code:
- Discussion: A hospice should report on the hospice claim the principle diagnosis and all diagnoses related to the terminal illness and related conditions. A hospice agency that has an unusually high number of claims with a single diagnosis may indicate that the hospice is not reporting all related diagnoses.
- Concern: Is the hospice agency meeting Medicare CoP and able to offer all four levels of care?
- Target Area: No general inpatient or continuous home care
- Discussion: Medicare Conditions of Participation require hospices to demonstrate they can provide all four levels of care: routine home care, general inpatient care, inpatient respite care, and continuous home care to be a Medicare certified hospice provider. A report published by CMS in 2014, included an analysis of 2012 hospice claims:
- 3% of beneficiaries did not have any general inpatient care in 2012
- 1% of hospice agencies did not provide any general inpatient care to any of their patients
- 4% of all hospice days billed in 2012 were billed as continuous home care
- 7% of hospice agencies billed at least one day of continuous home care but the share of continuous home care days billed varied across hospice agencies that billed any continuous home care days
- Almost 90% of hospice agencies that provided continuous home care had less than 1% of their days billed as continuous home care
- Four hospices billed more than 10% of their days as continuous home care.
This target area aims to monitor that hospice agencies are meeting the hospice Conditions of Participation and are able to provide all four levels of care.
- Concern: Is general inpatient care being used appropriately and are patient symptoms being managed well?
- Target Area: Long general inpatient stays
- Discussion: General inpatient stays are intended to be short term, to treat acute symptoms. If a patient has extended general inpatient stay there this level of care is not being used appropriately or that symptoms are not being properly managed.
- Concern: Is Medicare making prescription drug Part D payments when these should be paid by the hospice agency?
- Target Area: Medicare Part D payments for hospice beneficiaries
- Discussion: Hospice agencies are paid a per diem rate for each day that a patient is in hospice care, irrespective of the services that the hospice agency provides to the patient. Drugs that the hospice agency provides to the patient are included in the hospice rate. In 2019, CMS released a report analyzing 2016 Medicare Part D payments being made for beneficiaries on hospice care. Part D is the Medicare prescription drug plan. The study focused on four categories of drugs that are often prescribed to patients at the end of their lives as well as two disease specific drugs for two diseases. Part D should not pay for drugs if the patient is on hospice and the drug is covered under the hospice benefit. Based upon sample results, CMS estimates that Medicare paid $160.8 million for drugs that hospice agencies should have paid for, constituting and overpayment to hospice.
How can a hospice use this information?
With a better understanding of the underlying motivation for each of these target areas, a hospice agency should carefully look at the data on its PEPPER reports to identify any metrics that indicate a need for further investigation and possible process improvements. These reports are a powerful way to benchmark a hospice agency’s performance relative to itself (over a running three year period) as well as relative to other hospice agencies – across the nation, in its state, and in its MAC jurisdiction.
Where can you get more information?
by editor | Nov 13, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
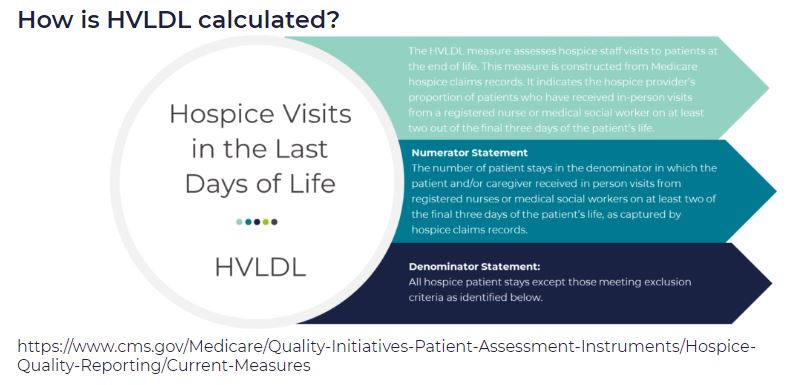
HVLDL is an HQRP claims-based measure of the proportion of patients who have received in-person visits from a registered nurse (RN) or a medical social worker (MSW) on at least two of the final three days of life. This metric replaces the HIS-based measure Hospice Visits When Death is Imminent (HVWDII).
CMS selected this metric as an important measure of quality since it is during these final days that patients most likely exhibit extreme symptoms of actively dying. This time period is also when patients most often exhibit signs of onset of clinical signs of dying. Finally, consistent visits in the final days of life are perceived as better level of care by the patient’s family.
How are the final three days of life defined?
For the purposes of HVLDL, the final three days of life are defined as:
- Day 1: day of death
- Day 2: day prior to death
- Day 3: day two days prior to death
How are days counted?
- This metric counts days, not visits
- If an RN and a MSW each visit the patient on the same day, this counts as a single day not as two visits, since the metric counts days not visits
- Telephonic visits do not count toward this metric, only in person visits
- Visits by LPN, chaplains, or other clinical staff do not count toward this metric
Which patients are included in the calculation of HVLDL?
All Medicare fee for service hospice patients are included in this metric with the following exceptions:
- Patients who did not die in hospice care
- Patients who received continuous care, respite care, or general inpatient care in the final three days of life
- Patients who were enrolled in hospice care for fewer than three days
Since HVLDL measures visits over the final three days of life, a patient must have been enrolled in hospice for at last three days to be included in the metric.
What are the data sources for this metric?
Data for HVLDL is calculated from Medicare claims data. Only data for Medicare fee for service patients who died while in hospice and who do not meet any of the exceptions listed above are included in the HVLDL calculation.
CMS calculates HVLDL using eight consecutive quarters of data. Hospice agencies with fewer than 20 “eligible patients” in the reporting period (where an “eligible patient” is defined as a patient who has died while under hospice care and does not fall under any of the exceptions listed above) are not assigned an HVLDL value. By including eight quarters of data, CMS is expanding the set of hospice agencies for which an HVLDL value will be reported. CMS will update the HVLDL value once each year.
How is the HVLDL metric calculated?
- The denominator is the count of all “eligible patients” during the reporting period
- The numerator is the count of all “eligible patients” who received an RN or MSW visit on at least two of the three final days of life
When was HVLDL introduced and where can patients and their families view the HVLDL value?
HVLDL was added to the HQRP in 2021 and began public reporting in 2022. The metric provides insight into care provided by the hospice agency in the days immediately leading up to patient death. HVLDL can be seen under the Quality of Patient Care section on the Care Compare website.
How can a hospice see its HVLDL value?
To support a hospice agency’s quality improvement efforts, CMS shares the agency’s HVLDL value in the Hospice Agency Level QM Report in CASPER. CASPER reports separately the numerator and denominator of HVLDL as well as the hospice observed percent – the agency’s HVLDL score. CASPER also reports on the national average HVLDL score and the agency’s percentile. Percentile rank indicates what percentage of agencies nationwide had a HVLDL score that was equal to or lower than the agency’s score. A hospice agency can benchmark its HVLDL score with the national average and the percentile rank. It can also trend its performance against its own HVLDL value over time.
Why did CMS replace the HIS HVWDII?
CMS implemented HVWDII in 2017. This metric measured hospice visits by non-clinical team members including LPN, chaplain, MSW, and hospice aides during the final seven days of a patient’s life. Analyzing the data collected by this metric, CMS found that HVWDII was unable to distinguish between high quality and low quality hospice agencies (i.e., it failed the CMS validity testing criteria). Consequently, CMS sought a replacement metric. The revised metric is also aligned with the Service Intensity Add-On (SIA) payment initiative (which incentivizes visits by RN and MSW near patient’s death). HVLDL has an added benefit that it is calculated based on claims data so it does not add a reporting burden for hospice agencies.
Where can you learn more?
Image from Medalogix
by editor | Nov 13, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
HCI is a single comprehensive metric reflecting ten indicators of care delivered during a hospice stay — from admission to discharge. This metric, which is included in the patient Care Compare portal, is intended to provide patients, families, and caregivers with an added metric to support informed healthcare choices.
What are the data sources for this metric?
HCI is calculated from Medicare claims data. A hospice agency does not need to submit any additional data to CMS for the calculation of this metric. The HCI metric captures care processes throughout the duration of a patient’s hospice care – from admission through discharge. Only data for Medicare fee for service patients who have been discharged from hospice is included in the HCI metric. CMS calculates HCI using eight consecutive quarters of data. Hospice agencies with fewer than 20 discharges in the reporting period are not assigned an HCI value. By including eight quarters of data, CMS is expanding the set of hospice agencies for which an HCI value will be reported. CMS will update the HCI metrics once each year.
What does the HCI metric measure?
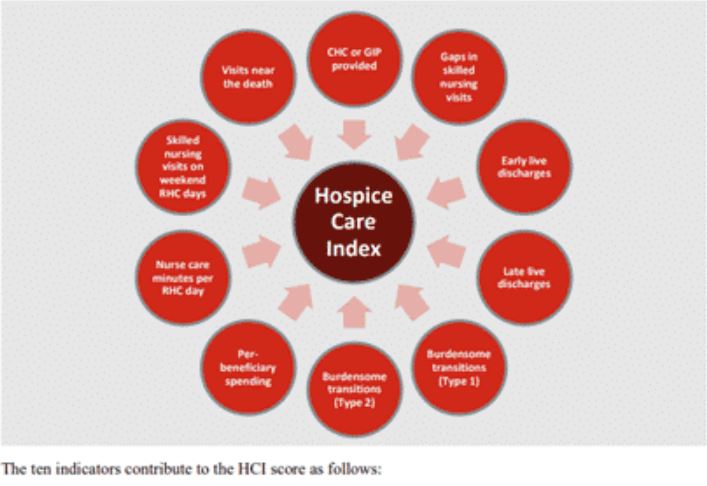
HCI is a single comprehensive metric comprised of the following ten individual indicators of care.
- CHC/GIP provided
- Gaps in skilled nursing visits
- Early live discharges
- Late live discharges
- Burdensome transitions (Type 1)
- Burdensome Transitions (Type 2)
- Per-beneficiary Medicare spending
- Nurse care minutes per routine home care days
- Skilled nursing minutes on weekends
- Visits near death
Each indicator measures a different aspect of hospice care. A set of the HCI indicators measure the agency’s provision of higher level of care as needed and more frequent visits closer to the time of death, as measured by indicators
- Gaps in skilled nursing visits
- Nurse care minutes per routine home care day
- Skilled nursing minutes on weekends
- Visits near death
A set of HCI indicators measure patterns of live discharges and transitions, as measured by indicators
- Discharges from hospice followed by hospitalization and hospice readmission
- Discharge from hospice followed by patient dying in the hospital
- Early live discharges
- Late live discharges
Finally, an HCI indicator is used to measure appropriateness of use of the hospice benefit
- Per beneficiary Medicare spending
Medicare’s overall objectives of the HCI metric are twofold: (i) to ensure that all hospice patients are receiving the care that they need and (ii) to identify indicators of fraud.
How is the HCI metric calculated?
The HCI metric simultaneously monitors all ten indicators of care. The ten indicators are then combined into a single value between zero and ten, where ten is the highest value. Each indicator equally affects the HCI value, reflecting how each aspect of care delivered, from admission to discharge, shares the same level of importance.
Specifically, the hospice agency is awarded one point for each of the ten indicator criteria the agency meets. A hospice receives a point for an indicator if its value exceeds a prescribed threshold. The threshold is determined as a function of the overall values for that indicator across all hospice agencies. The more indicators a hospice agency meets, the higher the agency’s HCI value. The sum of the points earned from meeting the criterion for each indicator yields the agency’s aggregated single HCI value.
When was HCI introduced and where can the metric be viewed?
The HCI metric was added to the HQRP and began public reporting in 2022.
The single aggregate HCI metric can be seen under the Quality of Patient Care section on the Care Compare website.
The details of the HCI metric – including the values for each of the ten individual HCI indicators – can be found in the Provider Data Catalog.
How can a hospice see details about its HCI value?
To support a hospice agency’s quality improvement efforts, CMS shares the details of an agency’s HCI indicator scores in the Hospice Agency Level QM Report in CASPER. An agency can benchmark its indicator values with state and national averages. It can also trend its performance in each indicator over time.
Where can you learn more?
Image from Home Care Pulse






