
by editor | Oct 13, 2024 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Interdisciplinary Team, Regulatory Compliance, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Social Workers
The hospice interdisciplinary group (IDG) creates a patient’s plan of care and provides holistic care to the patient, caregiver, and family. Hospice Conditions of Participation require the IDG to “review, revise, and document the individualized plan as frequent as the patient’s condition requires, but no less frequently than every 15 calendar days.”
As such, the IDG meet at a minimum every 15 days. In many hospice organizations, the interdisciplinary group meets weekly to review patient status and to determine if changes are required to a patient’s plan of care. It is important that during the IDG meeting patients’ care plans are reviewed and updated based upon patients’ assessments. Timely and accurate documentation is critical; this documentation may be reviewed by surveyors and by CMS to ensure compliance with regulations.
Who is required to attend an IDG Meeting
Required members of the IDG meeting include:
- A doctor who is an employee or under contract with the hospice agency
- Registered nurse
- Social worker
- Pastoral or other counselor
These four individuals are minimum participants in the IDG meeting. If one of these members i missing from the IDG meeting, the meeting does not meet Medicare regulations and it is considered as if the meeting did not take place. . Care must be taken to ensure that the minimum requirement – IDG meeting with the participation of at least these four individuals at a minimum of once every 15 days – is met.
Additionally, a staff member is typically identified to serve as the scribe for the IDG meeting. The scribe captures any changes to a patient’s plan of care that are agreed upon during the meeting.
What activities occur during the IDG meeting?
When the meeting begins, all participants sign the meeting sign-in sheet. These sheets serve as documented proof that the hospice has met the Medicare Conditions of Participation – that the required members of IDG participated in the meeting. Sign in sheets are stored in a place that is accessible for review upon the request of auditors or surveyors.
Prior to the IDG meeting, a list is drawn up of the patients who will be reviewed during the meeting. For each of these patient’s members of the care team provide an update on the patient’s current condition, highlighting any concerns. The team then discusses the plan for the upcoming two weeks.
Patients may be ordered for discussion as follows:
- Deaths
- Admissions
- Recertifications
- Evaluation
Let’s review each of these in detail.
Deaths
Each death since the prior IDG meeting is reviewed. The team discusses whether bereavement has been requested or declined. In the case where bereavement has been requested, the individuals who will be receiving bereavement services are identified. Any further details or concerns on the services that will be provided are discussed.
Admissions
The RN Case manager discusses any new admissions since the prior IDG meeting, including patient diagnosis and hospice eligibility criteria. Visit frequency is discussed, hospice aide services, and patient psychosocial needs. Typically, all team members partake in this discussion including a discussion about patient medications and prognostic indicators.
Recertifications
At this stage in the IDG the team discusses all patients who are the end of their benefit period and need to be recertified. Any face-to-face visits that were conducted will be discussed and any that are still pending will need to be scheduled. For patients who were evaluated and are found not to meet criteria, the team discusses how to notify the family and details on how to transition the patient off of hospice care.
Evaluations
All remaining patients on the list are reviewed by the members of the IDG. The team discusses whether any changes to the plan of care are needed, whether any medications need to be changed or if any additional support is required (e.g., chaplain, volunteer). The plan of care may be updated if the team agrees that a change in visit frequency is required.
Updating patients’ plan of care
While each patient is discussed, any changes to the patient’s plan of care are entered into the patient’s chart, which is signed by the medical director.

by editor | Aug 25, 2024 | Care Keys - Aides, Care Keys - Chaplains, Care Keys - Nurses, Care Keys - Social Workers, Rules and Regulations - Office Team, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
As a member of the hospice healthcare team, you play an important role in caring for your patients. Because of this, you will often learn private information about them – not just about their health, but also about their personal relationships, their financial situations, and other sensitive and personal information. It is important to understand that you have a legal and ethical responsibility to keep this information confidential and only share it – when necessary – with other healthcare professionals who are part of the patient’s care team. It is your responsibility to protect patient privacy.
Why is it important to keep healthcare information private?
In 1996, the Health Insurance Portability and Accountability Act (HIPAA) was passed to protect people’s health information. The main goal of HIPAA is to ensure that health information is kept private and secure, and only shared with those who need to know in order to provide care or process medical records. This law applies to everyone working in healthcare.
What does HIPAA protect?
HIPAA protects what is called “personal health information” (PHI). This includes any details that could identify a patient, such as:
- Name
- Medical record number
- Date of birth
- Address
- Email address
- Social security number
Only those directly involved in a patient’s care or those who handle billing or administrative tasks should have access to this information.
Your role as a member of the patient’s healthcare team
As a member of the patient’s healthcare team, it is important to follow HIPAA rules to protect your patient’s privacy. If you share a patient’s health information without permission, it can harm the patient and break the trust they have in you. Here are some important things to keep in mind:
- Do not share information unnecessarily: Never discuss a patient’s health with friends, family, or on social media. Only discuss patient care with other healthcare workers who are directly involved in that patient’s care.
- Keep conversations private: If you need to talk about a patient’s care with another healthcare worker, make sure you do so in a private place where others cannot overhear.
- Secure patient records: Whether you are handling paper records or using electronic systems, always ensure that patient information is stored securely.
Why following HIPAA is important
By following HIPAA regulations, you help protect your patient’s privacy, ensure their information is handled with respect, and build trust. Patients and their families rely on you to keep their personal information safe, and HIPAA provides the guidelines you need to do so.
What are the guidelines of not following HIPAA?
Hospices and their employees must protect patient information at all times. If HIPAA rules are not followed, it can lead to serious consequences including fines, penalties, and even imprisonment. This applies not just to the hospice itself but also to any vendors or contractors who work with patient information.
Final thoughts
Understanding and following HIPAA is an essential part of your job as a member of a patient’s healthcare team. By keeping patient information private, you help ensure their safety, comfort, and trust in the care they receive. Remember, protecting privacy is not just a legal requirement – it is a crucial part of providing compassionate and respectful care.
Where can you find more information
by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
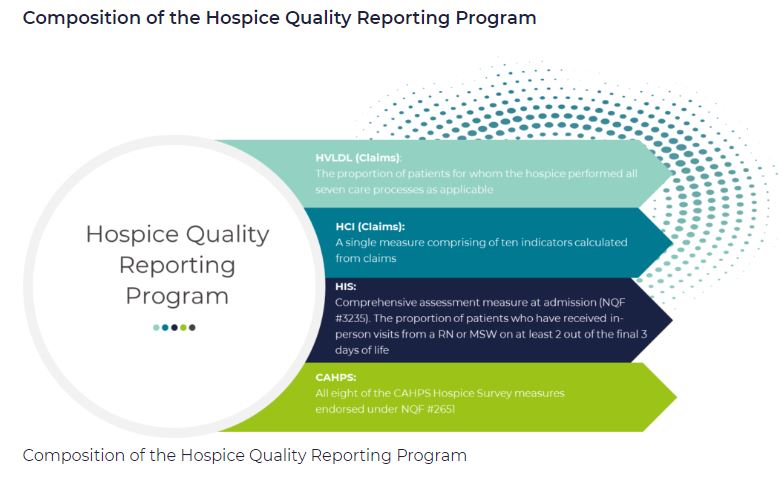
Which measures are included in HQRP?
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
All Medicare certified hospice agencies must submit an HIS Admission and HIS Discharge record on all admissions and discharges from their agency. The report must include all patients, irrespective of payer source, patient age, or location where hospice services were provided. It is recommended that data is submitted within 14 days to be sure that it is accepted within the required 30 day time frame. Submitting early will give the hospice agency time to adjust and correction the data, as needed.
What information is included in each HIS record?
- Admission HIS: Captured during the admission process
- Administrative information
- Preferences
- Active diagnoses
- Health conditions
- Medications
- Record administration
- Discharge HIS: sections of information captured during the discharge process
- Administrative information
- Service utilization (this has been replaced)
- Record administration
How are HIS records used?
The HIS record is used to compute seven process measures:
- Patient treatment preferences
- Beliefs/values address if desired by the patient
- Pain screening
- Pain assessment
- Dyspnea treatment
- Dyspnea screening
- Patients treated with an opioid who are given a bowel regimen
These process measures are combined to compute a single composite quality measure – the Comprehensive Assessment at Admission – that is reported on Care Compare. This composite measure assesses whether the seven key care processes were followed when a patient was admitted to hospice.
What are HIS Submission requirements?
- Within 30 days of patient admission or discharge of each hospice patient. All HIS records must be successfully accepted by QIES ASAP system within 30 calendar days of the patient admission or discharge date. See here for details on submitting HIS data
- – The requirements have included an incrementally increasing compliance threshold since data collection began. The Final Rule stated that beginning with FY 2018 reporting year, to avoid the 2 percentage point reduction in Annual Payment Update (APU), hospice agencies were required to submit at least 70% of their required HIS records within the 30 day deadline. For FY 2019 this minimum threshold was increased to 80% of all required HIS records. For FY 2020 and all subsequent years, the minimum threshold was increased to 90% of all required HIS records within the 30 day deadline. Hospice agencies that meet the submission threshold will avoid the 2% reduction in APU payment.
- – Non compliant providers, that is – providers that fail to meet this submission threshold, receive notification from CMS via a HQRP non-compliance letter that CMS sends via USPS and via the CASPER system. The CASPER letter identifies why the hospice agency is non-compliant and also provides information on how the hospice agency can request reconsideration. Agencies should monitor CASPER for receipt of such notice; agencies have 30 days from the date that the letter is sent for reconsideration.
How can a hospice agency validate that its HIS data has been accepted?
An agency can use reports in CASPER to monitor the status of HIS records submitted to QIES ASAP and track HIS record status, determine when correction of errors is required.
- The Hospice Timeliness Compliance Threshold Report enables a hospice agency to check the timeliness of acceptance of HIS records including the percentage of records that were submitted within the 30 day deadline to determine whether the agency will meet the required threshold.
- The Hospice Final Validation Report provides information on the status of submitted HIS files, indicated whether or not the records were accepted and details of any warning or error messages, if generated.
Where can you get more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
The CAHPS survey is intended to measure the experience of patients who had died while receiving hospice care and the experience of their primary caregivers. It surveys informal caregivers – usually family members – of the persons who died under hospice care.
The survey is a component of the Hospice Quality Reporting Program (HQRP). It is an experience survey rather than a satisfaction survey. The intention of this survey is to provide data that can be publicly reported on Care Compare. It is also intended to provide hospice agencies with data for quality improvement.
How and when is the survey conducted?
To give the caregiver some time for recovery, the survey is administered to the primary informal caregiver of those who died while receiving hospice care at least two months following the month of the patient’s death.
The survey is conducted by mail, by telephone, or by mail with telephone follow up, at the hospice agency’s preference.
How are survey responses reported on Care Compare?
The survey is comprised of 47 questions. Not all of the respondents answer all of the questions and not all of the survey responses are publicly reported. Instead, some of the responses are aggregated together to generate a composite measure that is reported to the public. The result is that eight measures are publicly reported: six composite measures comprised of responses aggregated across multiple questions and two single item measures.
Composite measures:
- Communication with the family
- Receiving timely help
- Treating the patient with respect
- Emotional and spiritual support
- Help for pain and symptoms
- Training the family to care for the patient
Single item measures
- Ratings of the hospice
- Willingness to recommend the hospice
Which hospice agencies must participate in the CAHPS Survey?
Any Medicare certified hospice agency that served at least 50 survey eligible hospice patients in the previous calendar year and that received its CCN after January 1 of the previous calendar year is required to participate in the CAHPS Hospice Survey. The hospice agency is required to successfully submit 12 months of data, from January through December, of the data collection year. Failure to participate will result in a 2% penalty from Medicare payments.
Which agencies are exempt from participating in the survey?
- Newness Exemption: A hospice that receives its CCN on or after January 1 is eligible for a one-time exemption from the CAHPS survey for the remainder of that calendar year. For example, a hospice agency that receives its CCN in 2022 will be required to participate in the CAHPS survey beginning with patients who die in January 2023, unless the agency meets the Size Exemption
- Size Exemption: A hospice agency can apply for an exemption from the CAHPS survey if the agency served fewer than 50 survey eligible patients or caregivers in the prior calendar year. If multiple facilities share a single CCN, the survey eligible patients count is the total from all facilities that share the same CCN. The form to apply for the exemption, submission deadline, and further details on exemption, can be found here
Who administers the CAHPS Hospice Survey?
A hospice agency is not permitted to directly administer the CAHPS hospice survey. Instead, the agency is required to use a CMS approved survey vendor to administer the CAHPS surveys on an ongoing monthly basis.
Where are these results reported?
All eight CAHPS quality measures are publicly reported. They are all also available in the CASPER Preview Reports so that a hospice agency is able to review the data before it is publicly reported on Hospice Care Compare.
May a hospice communicate with its patients and their caregivers about the survey?
If a hospice agency wishes to let its patients know about the CAHPS survey, it must notify all patients about the survey rather than selectively notifying patients. Additionally, the agency cannot try to influence the survey responses or ask caregivers to give certain ratings.
How does CMS adjust the data that is submitted?
The data is case mixed adjusted. That is, CMS tries to remove the effects that arise from the demographics of the patients served by each hospice agency. The intent is to make the scores more comparable across hospice agencies. Data that a hospice agency may receive from its survey vendor may not be case mix adjusted. Consequently, the data that a hospice agency receives from its survey vendor may not match the data that it sees in the CASPER Preview Report or on Hospice Care Compare.
Can a hospice agency review its data before it is publicly reported?
CMS provides a 30 day review period during which providers can use the Hospice CAHPS Provider Preview Report to review their CAHPS data before it is publicly reported on Care Compare. This report can be accessed on CASPER. If a hospice agency finds an error in the data after review the Preview Report, it may request that CMS review the data by submitting a request to the following address hospicecaphssurvey@hsag.com. However, all requests for review must be submitted within 30 days of release of the Preview Report. Detailed instructions for requesting a review of the data can be found here
Where can you find more information?

by editor | Nov 26, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Social Workers
Program for Evaluating Payment Patterns Electronic Reports (PEPPER) is a data analysis report that has been available for hospices since 2012. This report contains claims data statistics based upon UB-04 claims data submissions for a single hospice agency. The statistics are generated for areas that are that are targeted by CMS as at risk for potential of improper Medicare payment. The data is reported in tabular format as well as in graphical format showing a time series of the hospice agency’s performance over time.
What timeframe of data is included in the PEPPER report?
Each PEPPER report summarizes claims data for the most recent three fiscal years (October 1 through September 30).
When is the PEPPER report generated?
The PEPPER report is generated each April. The PEPPER distribution schedule, portal access instructions, and training and resources can be seen here. A PEPPER report is generated for each hospice agency, regardless of whether the agency’s data is concerning or the agency is targeted for additional medical review.
What are PEPPER target areas?
Target areas may change or be added if new areas are identified as target areas for potential risk of improper payment. The report is used by medical reviewers to determine whether a hospice agency should be targeted for additional medical review audits. Hospice PEPPER target areas are the following:
- Live discharges no longer terminally ill: percentage of all hospice patients who were discharged (by death or alive), who were discharged alive excluding patients who were discharged alive due to transfer, revocation, discharge for cause, or patients who moved out of service area
- Live discharges – revocations: percentage of all patients who were discharged (by death or alive) who were discharged alive due to patient revocation
- Live discharges with length of stay between 61-179 days: percentage of all patients who were discharged alive who had a length of stay between 61-179 days
- Long length of stay: percentage of all patients who were discharged (by death or alive) who had a combined length of stay that exceeded 180 days
- Continuous home care provided in assisted living facility: percentage of patients living in assisted living facility for any portion of episode who were discharged (by death or alive) where at least eight hours of continuous care were provided while the patient was in an assisted living facility
- Routing home care provided while patient in assisted living facility: percentage of all routine home care days provided by hospice that were provided while patient was in assisted living facility
- Routing home care provided while patient in nursing facility: percentage of all routine home care days provided by hospice that were provided while patient was in nursing facility
- Routing home care provided while patient in skilled nursing facility: percentage of all routine home care days provided by hospice that were provided while patient was in skilled nursing facility
- Claims with single diagnosis code: percentage of all claims submitted that have a single diagnosis code
- No general inpatient care of continuous home care: percentage of all discharged patients (by death or alive) who had periods of general inpatient care that were longer than five consecutive days
- Average number of Medicare part D claims for beneficiaries residing at home: This metric only includes hospice episodes that are at least three days and that occur at home. For these, the average number of part D claims is computed
- Average number of Medicare part D claims for beneficiaries residing in assisted living facility: This metric only includes hospice episodes that are at least three days and where the patient resides in an assisted living facility. For these, the average number of part D claims is computed
- Average number of Medicare part D claims for beneficiaries residing in a nursing facility: This metric only includes hospice episodes that are at least three days and where the patient resides in a nursing facility. For these, the average number of part D claims is computed
How can a hospice agency use its PEPPER report?
An agency’s PEPPER report contains the agency’s statistics for the target areas as well as comparative statistics: national statistics, state statistics, and MAC jurisdiction statistics. The PEPPER report allows a hospice agency to compare its billing practices to those in its MAC jurisdiction, its state, and across the nation. The PEPPER report also compares the hospice agency’s target values to the national, jurisdictional, and state percentile 80th values for each target area. Agencies that fall above the 80th percentile in any of the target area are considered at risk for improper payment for that risk area. Agencies that score above the 80th percentile in one or multiple target areas will likely be subjects for additional medical review such as TPE, OIG review, or UPIC audit.
- A hospice can use the PEPPER Compare Targets Report to prioritize areas for audit or improvement. The Compare Targets Report reports the hospice agency’s percentile for each target area. A hospice agency can quickly identify if it is at high risk in any target area: if a hospice agency is above the 80th percentile in any target area, the percentile is printed in bold red. Otherwise, all percentiles are printed in black but the agency should check the percentile values. High percentile values (even if below 80) may indicate a potential risk area that the hospice agency should monitor.
- The target area graph shows a bar graph of the hospice agency’s target area value over the three years of data for each of the target areas. The graph also has three trend lines, one for each of the three 80th percentiles for each of the comparison groups. Significant changes in the values over the years should be investigated to be sure that the reason is understood. Is it due to change in staff? Change in management? Change in policies? Or, are there a possibility of improper payments?
- The target area hospice data table contains the various data elements that are used to compute the target area statistic.
- The comparative data table provides the 80th percentile values for the three comparative groups: nation-wide, MAC jurisdiction, and state. The value for any of the comparative groups will be reported as zero if there are fewer than 11 hospices in that group.
How can a hospice agency access its PEPPER report?
PEPPER reports are not sent to hospice agencies. A hospice agency can access its PEPPER report electronically at this website.
Should a hospice access its PEPPER report?
Hospices are encouraged to access their PEPPER reports. The metrics in the PEPPER can support hospice agencies with internal monitoring and auditing. Hospice agencies can use the data to detect trends over time and to compare their performance with that of other agencies – nationally and regionally — to identify outlier behavior and potential anomalies that require further investigation. Reviewing a PEPPER gives a hospice agency the opportunity to improve behaviors or take other corrective actions that can improve the outcome of an audit. It allows a hospice agency to implement self monitoring, self correction, and self improvement which is preferrable to waiting until CMS or a MAC identifies an issue. Additionally, if a hospice is under a large scale audit and has not accessed its PEPPER report, the government may claim that the issues were known or that the agency should have known that it had improper payment practices.
Where can you find more information?






