
by editor | Oct 13, 2024 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Interdisciplinary Team, Regulatory Compliance, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Social Workers
The hospice interdisciplinary group (IDG) creates a patient’s plan of care and provides holistic care to the patient, caregiver, and family. Hospice Conditions of Participation require the IDG to “review, revise, and document the individualized plan as frequent as the patient’s condition requires, but no less frequently than every 15 calendar days.”
As such, the IDG meet at a minimum every 15 days. In many hospice organizations, the interdisciplinary group meets weekly to review patient status and to determine if changes are required to a patient’s plan of care. It is important that during the IDG meeting patients’ care plans are reviewed and updated based upon patients’ assessments. Timely and accurate documentation is critical; this documentation may be reviewed by surveyors and by CMS to ensure compliance with regulations.
Who is required to attend an IDG Meeting
Required members of the IDG meeting include:
- A doctor who is an employee or under contract with the hospice agency
- Registered nurse
- Social worker
- Pastoral or other counselor
These four individuals are minimum participants in the IDG meeting. If one of these members i missing from the IDG meeting, the meeting does not meet Medicare regulations and it is considered as if the meeting did not take place. . Care must be taken to ensure that the minimum requirement – IDG meeting with the participation of at least these four individuals at a minimum of once every 15 days – is met.
Additionally, a staff member is typically identified to serve as the scribe for the IDG meeting. The scribe captures any changes to a patient’s plan of care that are agreed upon during the meeting.
What activities occur during the IDG meeting?
When the meeting begins, all participants sign the meeting sign-in sheet. These sheets serve as documented proof that the hospice has met the Medicare Conditions of Participation – that the required members of IDG participated in the meeting. Sign in sheets are stored in a place that is accessible for review upon the request of auditors or surveyors.
Prior to the IDG meeting, a list is drawn up of the patients who will be reviewed during the meeting. For each of these patient’s members of the care team provide an update on the patient’s current condition, highlighting any concerns. The team then discusses the plan for the upcoming two weeks.
Patients may be ordered for discussion as follows:
- Deaths
- Admissions
- Recertifications
- Evaluation
Let’s review each of these in detail.
Deaths
Each death since the prior IDG meeting is reviewed. The team discusses whether bereavement has been requested or declined. In the case where bereavement has been requested, the individuals who will be receiving bereavement services are identified. Any further details or concerns on the services that will be provided are discussed.
Admissions
The RN Case manager discusses any new admissions since the prior IDG meeting, including patient diagnosis and hospice eligibility criteria. Visit frequency is discussed, hospice aide services, and patient psychosocial needs. Typically, all team members partake in this discussion including a discussion about patient medications and prognostic indicators.
Recertifications
At this stage in the IDG the team discusses all patients who are the end of their benefit period and need to be recertified. Any face-to-face visits that were conducted will be discussed and any that are still pending will need to be scheduled. For patients who were evaluated and are found not to meet criteria, the team discusses how to notify the family and details on how to transition the patient off of hospice care.
Evaluations
All remaining patients on the list are reviewed by the members of the IDG. The team discusses whether any changes to the plan of care are needed, whether any medications need to be changed or if any additional support is required (e.g., chaplain, volunteer). The plan of care may be updated if the team agrees that a change in visit frequency is required.
Updating patients’ plan of care
While each patient is discussed, any changes to the patient’s plan of care are entered into the patient’s chart, which is signed by the medical director.

by editor | Oct 7, 2024 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Interdisciplinary Team
Hospice care is patient- and family-centered, where the patient’s and family’s preferences and needs drive the care plan.
The hospice interdisciplinary group (also referred to as Hospice IDG or IDG), also referred to as the interdisciplinary team (IDT) is a team of healthcare professionals who work together to create a plan tailored to the needs of hospice patients. The IDG is crucial because it reflects the fundamental principle of hospice care: a multidisciplinary and holistic approach to treating a patient. Hospice care is not just about managing medical symptoms; it involves addressing the physical, emotional, social, and spiritual needs of the patient and their family. This comprehensive care model requires combined expertise of different healthcare professionals working together as a cohesive team.
Multidisciplinary and 360-degree approach
The idea of a multidisciplinary approach is central to hospice care because a single healthcare professional cannot fully address the complex needs of a patient at the end of life. Hospice patients often experience pain, emotional distress, social isolation, and spiritual concerns, all of which need to be treated so that the patient has a peaceful and dignified end of life experience. Each of the members of the IDG can address different aspects of hospice patient needs.
Physical needs: Managed by the physician and nurse. The physician provides medical direction and oversees patient care while the nurse manages the patient’s medical needs such as pain control and symptom management.
Emotional and social needs: The social worker provides emotional and social support, caring for emotional health, caregiver stress, and family dynamics. Consideration is also given to connecting the family with community resources
Spiritual needs: These are managed by the chaplain, who offers spiritual care and counseling, based on the patient’s and family’s beliefs. The chaplain helps patients and families explore spiritual concerns, questions of meaning, or religious beliefs in the context of their journey.
Daily living needs: Hospice aides assist with personal care like bathing, dressing, and grooming. They ensure dignity and comfort in activities of daily living like bathing, dressing, and grooming.
Companionship and support: The hospice volunteer offers companionship and practical help, like errands or respite for family caregivers.
By involving individuals from different disciplines, hospice care can take a 360-degress approach to a patient’s needs. It means that every aspect of care – physical, emotional, social, and spiritual – is addressed by someone with the expertise to manage that particular dimension. This all encompassing approach is what makes hospice care unique and effective.
Are all member of the IDG required per CMS regulations?
Per CMS regulations, only core members must always be part of the IDG to ensure that hospice care addresses every critical aspect of the patient’s experience. Four disciplines are considered core required members of the team. These include:
- Physician
- Registered nurse
- Social worker
- Chaplain
Some professional members may be included in the IDG as needed, depending upon patient’s individual circumstances. These include:
- Hospice Aide
- Volunteer
- Therapists
- Bereavement Counselor
How is the IDG aligned with regulatory standards?
CMS requires that hospice care involve an interdisciplinary approach because it reflects the need to treat the “whole” patient, not just their medical condition. The IDG ensures that the care plan is tailored to the patient’s evolving needs and that it incorporates feedback from multiple disciplines to achieve the best outcomes. The interdisciplinary model is also a regulatory requirement under the hospice Conditions of Participation (CoPs). As such, surveyors will review the functioning of the IDG during inspections to ensure compliance. A well coordinated interdisciplinary team ensures regulatory compliance and quality patient care.
Why is the interdisciplinary hospice team essential?
Hospice care is patient and family centered, meaning that the patient’s and family’s preferences and needs drive the care plan. The IDG works collaboratively to ensure that the care plan remains flexible and responsive to changes in the patient’s condition. As hospice patients often experience rapid changes in health, having professionals from different disciplines ensures that all aspects of care can be addressed promptly and effectively.
In summary, the IDG reflects hospice’s holistic, multidisciplinary approach to care by ensuring that all dimensions of the patient’s well-being are addressed. Required team members focus on medical, emotional, and spiritual care, while optional members can be added to meet unique or additional needs. This alignment ensures that hospice remains flexible and patient-centered.

by editor | Jan 29, 2023 | Billing, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Intake, Rules and Regulations - Nurses, Rules and Regulations - Office Team
What is the False Claims Act?
The False Claims Act (FCA) was established in 1863 during the Civil War to combat fraud and abuse perpetrated by suppliers of the federal government. At that time, the law was referred to as “Lincoln’s Law.”
The FCA has evolved significantly in recent years and is now one of the main tools used by the government to fight fraud. The FCA penalizes individuals or entities that submit fraudulent claims to the government, cause fraudulent claims to be submitted, or conspire to submit fraudulent claims.
One of the noteworthy provisions of the FCA is the qui tam provision, also known as the whistleblower provision. The qui tam provision allows private citizens, also referred to as “relators”, to report details of alleged fraud to the government. The whistleblower “stands in the shoes” of the government to prosecute the claim. This action benefits the government and the taxpayer as well as potentially the relator, who may receive a share of what is recovered.
How does the FCA relate to a hospice agency?
The False Claims Act allows hospice agency employees, patients, families of patients, or any individuals with alleged knowledge of fraud or abuse by the agency to report the behavior. Under the qui tam provision of the FCA, the relator may be entitled to a percentage of recovered funds.
What are different types of false claims?
A claim is a request for money made to the government. A false claim is money that is obtained from the government due to false or fraudulent claims. False claims include claims where the service
- Has not been provided
- Is already included as part of a different claim (i.e., double billing)
- Is not coded correctly
- Is not supported by the patient’s medical record
Claims may also be false and are covered under the FCA if they result from a referral made in violation of the Federal Anti-kickback statue (Stark Law).
The False Claims Act also includes payment from the government based upon false certification.
False claims include claims that the hospice agency should have known were false or fraudulent.
What is a claim that a hospice agency “should have known” is false?
The FCA expressly includes claims that a hospice agency “should have known” were false or fraudulent. “Should have known” means deliberate ignorance or reckless disregard of truth. As such, a hospice agency cannot avoid liability by simply ignoring inaccuracy in their claims. Examples of “should have known” include:
- Ignorance of billing rules, i.e., lack of knowledge of the rules
- Failure to act on consistent trends that are indicative of inaccurate billing
- Failure to act on inaccuracies or system errors identified by outside or internal auditing teams
- Failure to correct inaccurate billing (impacting either past or future claims)
A hospice agency must understand the rules and take proactive measures — such as conducting internal audits within the organization — to ensure compliance and accurate billing.
How can False Claims Act matters be initiated?
There are two ways that FCA matters can be initiated:
- Initiated by the government: When a FCA matter is initiated by the government, this type of matter typically starts with an audit or an investigation by the government. The government would determine that there is a false claim made to it and would initiate a matter, usually by a subpoena or civil investigative demand (CID). The government would issue the CID directly to the hospice agency. CID is a form of subpoena that requires the hospice agency to engage in one-sided discovery. That is, the hospice agency is required to produce documents demanded, respond to interrogatories, and provide sworn oral testimony. However, the hospice agency may not conduct any discovery.
- Qui tam matter: this type of matter is initiated by a whistleblower, also known as a “relator,” typically through the filing of a sealed lawsuit in a federal district court. The hospice agency does not know about the qui tam lawsuit since the lawsuit is initially served on the government. The case remains under seal while it is investigated by the government.
What is the qui tam process?
Qui tam actions are initially filed under seal. That is, only the US Attorney and some members of the Department of Justice (DOJ) have knowledge of and access to documents related to the case. The relator serves the complaint on the government together with a written disclosure of all material evidence.
The purpose of the sealed qui tam action is to allow the DOJ time to evaluate the relator’s allegations and for the DOJ to decide whether it would like to take over primary responsibility for prosecuting the case. If the DOJ decides to take over primary responsibility for the case, the DOJ is said to “intervene.”
The complaint remains under seal for 60 days during which time the DOJ investigates the relator’s allegations. This 60-day period can be (and typically is) extended. In fact, the government may spend months – or even years – investigating the case.
While the DOJ conducts its investigation, it may issue a Civil Investigative Demand (CID). This form of subpoena requires the defendant (the hospice agency) to engage in one-sided discovery where the hospice agency must produce documents, respond to interrogatories, and provide sworn oral testimony, as demanded. The CID is “one-sided discovery” because the hospice agency may not conduct any discovery.
If the government decides to intervene, the government is then responsible for litigating the case and files its own complaint instead of the complaint that was filed by the relator. The relator remains a party to the complaint.
If the government declines to intervene, the relator may proceed in her own name subject to the government’s right to dismiss the claim or to intervene at a later date.
Whether or not the government decides to intervene, the government remains the real party of interest. (As a reminder, the relator is only “standing in the shoes” of the government.) As such, the government must agree to any decisions on the case. The relator may not agree to dismiss or settle the case without the government’s approval.
What are the key phases in a False Claims Act investigation?
- Phase 1: FCA investigation is triggered. Triggers may include:
- Qui tam (whistleblower) lawsuit
- Call to OIG hotline
- Information identified during audit or claim review
- Complaints
- Data mining
- Phase 2: Formal investigation launches. Investigation may involve:
- Review of corporate filings
- Interview current or former employees
- Review financial records
- Electronic surveillance
- Physical surveillance of employees or of company premises
- DOJ civil investigative demand (CID), or the like
- Government search warrant or raid
- Phase 3: Litigation or resolution
Who are common whistleblowers?
Anyone can be a whistleblower and anyone may report alleged fraudulent activity to the government. The most common relators are:
- Business partners
- Current or former employees
- Competitors
- Patients
- Individuals who mine CMS data to identify anomalies/FCA claims
How can a hospice agency reduce the chance of qui tam lawsuits?
Any complaints or concerns that are raised – by employees, vendors, patients, or competitors, or any other individuals should be investigated and treated with concern as these have the potential to reveal compliance issues that need to be resolved by the hospice agency.
Employee complaints – whether from departing or active employees – are often an excellent source of information on potential compliance issues. A hospice agency should have a clearly established method – that is clearly and often communicated to employees – for employees to raise concerns. It should also have an organized process to diligently investigate and address any concerns raised by employees.
- Internal complaints:
- There must be an organized process – that is communicated regularly to employees – for employees to raise concerns
- All concerns must be investigated
- Have a plan to address any issues that are identified
- Take any necessary corrective actions
- Follow up with the individual who raised the complaint
- Provide training, as needed
- Departing employees
- Treat employees fairly as they leave
- Conduct exit interviews to identify any potential compliance concerns – investigate any issues that may be identified
- Potential releases (e.g., recovery from FCA claims)
Employees must feel that there is a process for raising concerns and that their concerns are heard. Employees should not fear retaliation for raising concerns. A hospice agency should be diligent and careful to respond to all employee complaints that are raised internally or to any complaints that are raised when employees leave the organization.
What are the financial benefits of avoiding FCA violations?
False claims act matters can be quite costly for a hospice organization. In addition to returning the payments associated with the false claims identified and incurring the costs associated with attorney fees to defend the matter, the hospice agency potentially faces the following significant costs:
- Treble damages: The FCA has a treble damages provision which provides that a hospice agency that is found to have violated the FCA statute may be liable to pay three times the amount of the actual false claim amount
- Penalty per claim: Under the FCA, a civil penalty may be assessed for each false claim that is submitted. The civil penalty dollar amount per claim has increased with inflation and currently may be as much as $23,000 per claim.
Where can you find more information?

by editor | Jan 29, 2023 | Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, QAPI, Regulatory Compliance
What is the governing body?
In accordance with the Conditions of Participation, a Medicare certified hospice agency must have a governing body. The governing body has ultimate responsibility for the hospice agency, including legal and financial authority. Medicare Conditions of Participation require that the governing body is informed of the ongoing activities at the hospice agency, including patient care delivery issues and all QAPI activities. The governing body must also appoint a qualified hospice administrator – a hospice employee with the necessary education and experience – who is responsible for hospice daily operations.
The governing body must meet at least quarterly and must maintain written minutes of its meetings.
There are two Conditions of Participation – 418.100 and 418.58 – that relate to the hospice governing body.
Condition of Participation 418.100
This Condition of Participation defines a standard that the governing body is responsible for management of the hospice agency, including its fiscal operations, provision of services, and continuous quality assessment and performance improvement (QAPI) efforts. The governing body also assumes full legal authority of all hospice operations. It further specifies that the governing body should appoint an administrator that reports to the governing body and who is responsible for hospice agency daily operations. The hospice administrator must be a hospice employee and must have necessary training, education, and experience. CMS does not specify the process by which an administrator should be selected by the governing body. If a hospice agency has multiple locations, the governing body is responsible for administration, supervision, and services for all locations as well as for any arranged services.
Condition of Participation 418.58
This Condition of Participation discusses requirements of a hospice agency’s QAPI program. The governing body must ensure that the hospice agency maintains and implements an ongoing quality improvement and patient safety program. Program performance must be monitored on a regular basis. Further, the governing body must ensure that one or more individuals are selected to lead the organization’s QAPI efforts.
The hospice agency’s organization documents must specify that the hospice governing body is responsible for the QAPI program. Additionally, the governing body specifies the frequency of data collection and level of detail of data collected by the QAPI program.
Are there any state regulations?
State hospice licensure regulations may impose additional requirements on the hospice governing body. They may also have specific requirements on the administrator that is selected by the governing body. A hospice is required to meet the most stringent requirements (whether state or federal).
Surveyors will check that all conditions are met. A hospice agency should maintain evidence of the governing body’s role and activities. Governing body authorizations and activities should be documented in governing body meeting minutes, company organization documents, and company policies and procedures.
Where can you find out more?
CMS Conditions of Participation – Governing Body
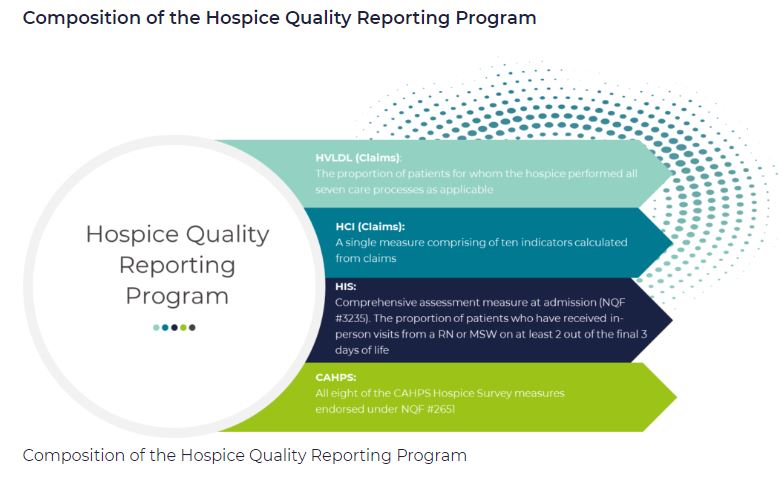
by editor | Dec 11, 2022 | Compliance and Regulatory - Directors, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Medical Records, Patient Care, Rules and Regulations - Nurses, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
What is the purpose of hospice quality reporting?
The Affordable Care Act authorized the establishment of a Quality Reporting Program for hospices. The Hospice Quality Reporting Program (HQRP) was established in 2014. HQRP aims to ensure that the level of quality in clinical care, symptom management, and patient and family experiences is at a high level across all hospice agencies. HQRP further aims to help patients and their families make informed decisions about end-of-life care. The measures and benchmarks reported in HQRP also provide CMS with measurements of hospice agency performance and how agencies are performing relative to other agencies in their region and across the nation. Some of the measures can also be used as indicators of Medicare fraud or abuse.
The Affordable Care Act also requires that quality measures relating to hospice care are reported on a CMS website.
HQRP data collection began in 2014 with two components. The first component was related to Hospice Item Set (HIS) data collection and transmission. The second component was related to the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Hospice Survey participation.
The Hospice Compare website was launched in 2017, enabling patients and their families to compare between the performance of different hospice agencies. In December 2020, Hospice Compare was replaced by Care Compare.
Which measures are included in HQRP?
HQRP measures care across a patient’s hospice stay. With a commitment to quality improvement, data transparency, and informed decision-making, the number of HQRP measures has increased since the launch of the program. As of 2022, HQRP includes four metrics, each of which includes several underlying measures:
What determines HQRP Compliance?
Performance level is not considered when determining compliance with HQRP; CMS requires a hospice agency to submit data completely, and on time, to be considered compliant. A Medicare-certified hospice agency is HQRP compliant if it submits the required data within the required timeframe and the data is accepted. A hospice agency is not compliant if it submits data but the data is not accepted. Failure to comply with HQRP requirements results in a two percentage point reduction in Annual Payment Update (APU). That is, for a hospice agency to preserve its full payment update, the agency must meet all HQRP data submission requirements. Failure to submit results will also impact an agency’s results on Care Compare.
How does CMS use the data that is submitted?
CMS currently uses the collected data internally for strategic planning purposes. CMS also uses the act of reporting to raise attention and awareness and promote actions to improve patient care.
Can a hospice agency verify its HQRP data before it is publicly published?
A hospice agency can review its HQRP data via the CASPER system before the results are made public on Care Compare. CASPER reports can be accessed by selecting the CASPER Reporting link to the CMS Quality Improvement and Evaluation System (QIES) Systems for Providers webpage. Hospice-specific reports are located in the Hospice Provider and Hospice Quality Reporting Program reporting categories in CASPER. Hospice agencies should review this data before it is published on Care Compare to ensure data accuracy, since the published data is used by the public to compare and select a hospice agency for end-of-life care.
Where can you find more information?

by editor | Oct 18, 2022 | Billing, Billing - General, Compliance and Regulatory - Directors, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Office Team, Hospice 101 - Social Workers, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Social Workers
To receive hospice services under the Medicare benefit, a patient or his authorized representative must elect hospice care.
If the patient or authorized representative elects to receive hospice care, the patient must file an election statement with a specific hospice agency. The election statement serves to indicate that the patient is choosing hospice care.
The election statement and the election statement addendum are conditions for payment.
What is the structure of a hospice election form?
Every hospice agency can design and create their own hospice election statement form although Medicare has published a model form that can be used by hospice agencies Model Hospice Election Statement. The election statement must include all of the following elements:
- Name of hospice agency that will be providing the services
- Acknowledge that nature of hospice services have been explained to the patient including, in particular, the palliative rather than curative nature of care
- Acknowledge patient understands that by electing hospice care, some Medicare services are waived
- For hospice elections beginning on or after October 1, 2020, a statement that although it would be rare, there could be some necessary items, drugs, or services that may not be covered by hospice because these items are deemed to be unrelated to the terminal illness or related conditions
- The effective date of the election. This may be the first day of hospice care of a later day. But it cannot be a date that precedes the date that the election statement was signed by the patient or their authorized representative.
- The individual who is serving as the patient’s attending physician, if any.
- Acknowledgement that the identified attending physician was the choice of the patient or authorized representative
- Signature of patient or authorized representative
There are some additional requirements for the election statements for elections beginning dated October 1, 2020 or later. These election statements must also include :
- Information on patient cost sharing for hospice services
- Notification of the patient or authorized representative right to receive an addendum to the election statement. The addendum is only required to be furnished to beneficiaries, their authorized representatives, non-hospice providers, or Medicare contractors who request this information. This addendum includes a list and rationale for the items, drugs, or services that are not covered by hospice services because the hospice has deemed these to be unrelated to the terminal illness and related conditions.
- Information on the Beneficiary and Family Centered Care Quality Improvement Organization (Beneficiary and Family Centered Care (BFCC) ), including that immediate advocacy is available through this organization if the patient disagrees with the hospice’s determination regarding non-covered services
Right to Request Patient Notification of Non-Covered Items, Services, And Drugs
At any time, a patient may request, in writing, the Patient Notification of Hospice Non-Covered Items, Services, and Drugs. addendum to the election statement.
The hospice agency must provide the notification within five days, if this request is made on the start of care date.
If the request is made during the course of hospice care, the hospice agency must provide the requested notification within 72 hours.
If the patient (or authorized representative) requests the addendum at the start of care but dies with five days, the hospice is deemed to have met its requirement and is not required to provide the addendum.
When would a hospice update the addendum?
The addendum lists the patient’s diagnoses and conditions that are present upon hospice admission and the items, services, and drugs that are not covered by the hospice because they are deemed to be unrelated to the terminal illness and related conditions.
During the course of hospice care, the addendum may require update, for example, if the patient’s plan of care is updated.
Changes to the addendum will need to be signed by the patient or his authorized representative and stored in the patient’s medical record with the hospice agency.
Where can you find more information on the election statement






