Root Cause Analysis (RCA) is a systematic and structured process used to identify the root causes that results in an undesirable outcome or adverse event and to develop corrective actions. The goal of RCA is not just to treat the symptoms of a problem but to delve into the underlying causes of the failure. By understanding these causes, a hospice agency can develop strategies to mitigate risks and implement corrective actions to prevent future occurrences. This approach to addressing adverse events will lead to improved patient safety and enhanced quality of care. Further, by promoting a culture of safety, RCA fosters a culture of transparency, accountability, and continuous improvement in the hospice agency.
When is root cause analysis used
A hospice agency can use root cause analysis to investigate any unexpected occurrences such as hospice acquired pressure ulcers, medication errors, or process variations where recurrence could result in serious adverse outcomes. Candidates for RCA may be identified via patient satisfaction surveys, incident reports, surveys, or other reports and audit activities. A root cause analysis focuses on systems and processes, rather than individuals in the agency. The objective of the RCA is to reduce the risk of recurrence by identifying opportunities to improve or redesign systems or processes. By implementing system changes, this will lead to sustained system improvement.

Elements of root cause analysis
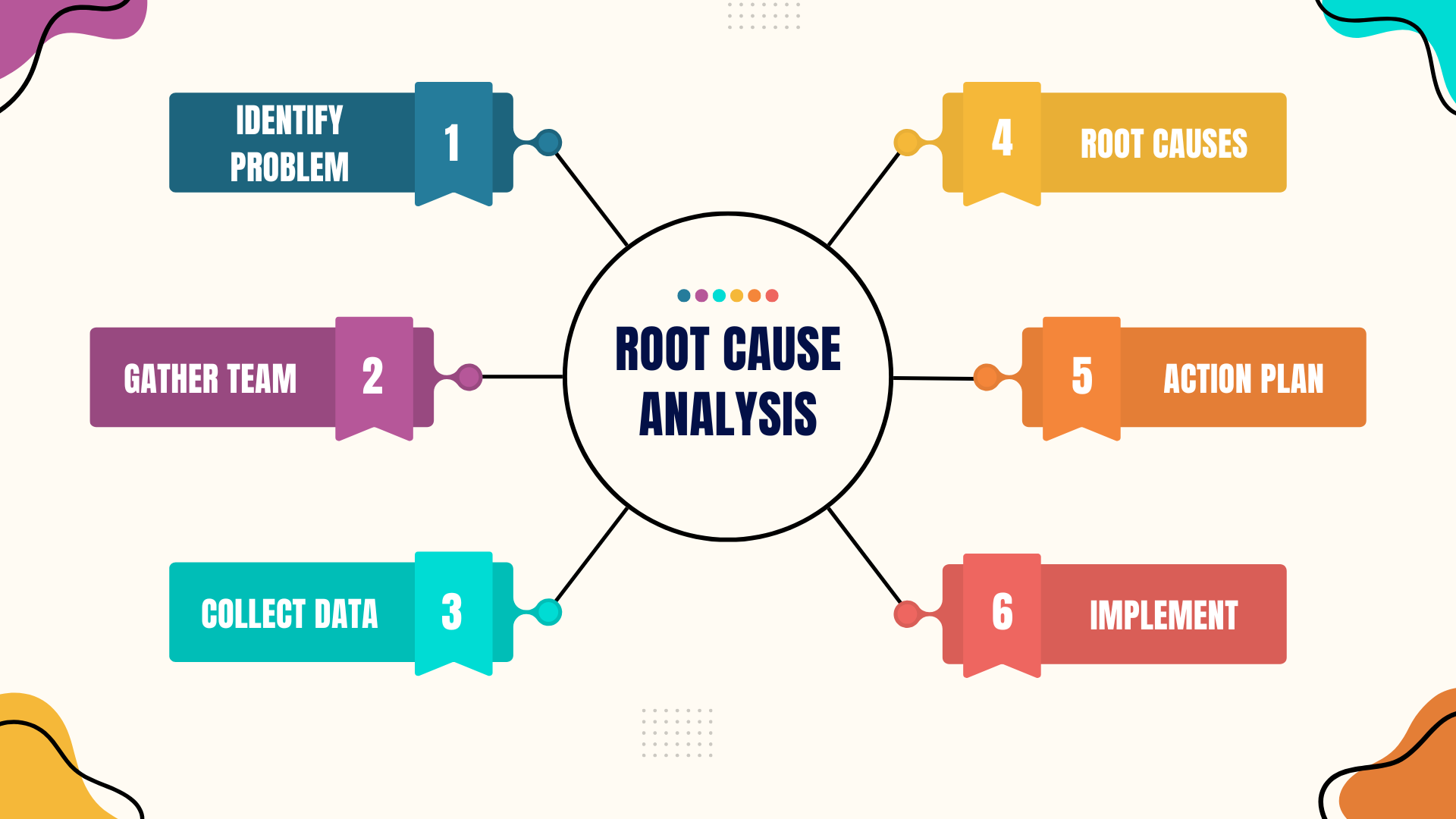
A comprehensive root cause analysis includes the following elements:
- Problem identification: Identify the adverse event to be investigated and gather preliminary information. Events and issues can come from many sources such as patients and their families, staff, or regulatory bodies. The agency should have a process for deciding what events are selected for RCA.
- Define the event: Clearly describe the adverse event, error, or near miss. Include specifics such as what happened, where, when, and who was involved.
- Gather initial data: Collect all relevant information about the event including incident reports, patient records, and witness statements.
- Assemble RCA team: Select the members of the RCA team and the team facilitator. Team members should be knowledgeable about the processes and systems that they will be investigating.
- Multidisciplinary team: Form a team that includes individuals with diverse expertise and perspectives. This may include clinicians, administrators, and support staff.
- Assign roles and responsibilities: Clearly define the roles and responsibilities of each team member to ensure an organized and effective RCA process.
- Data collection and investigation: Collect and organize the facts about the adverse event that will be investigated.
- Detailed event timeline: Create a detailed timeline of events leading up to, during, and after the incident. This helps in understanding the sequence of events.
- Conduct interviews: Interview staff involved in the event to gain insights into what happened and why. Ensure a non-punitive approach to encourage open and honest communication. Review documentation: Examine all relevant documentation including patient records, policy and procedure manuals, and any relevant logs.
- Identify contributing factors and root causes: Identify the situation, circumstances, or conditions that increased the likelihood of the adverse event. Conduct a thorough analysis of contributing factors that lead to identification of underlying process and system issues.
- Cause and effect analysis: Use tools such as fishbone diagrams (Ishikawa) or flow charts to map out the possible causes and identify the root causes. Five whys technique: Ask “why” repeatedly (usually five times) to drill down to the underlying root cause(s) of the problem.
- Develop action plan: Develop plan for best changing the processes and systems to reduce the likelihood of another similar event. Design and implement changes to eliminate the root causes. This may involve creating new processes.
- Corrective actions: Develop specific, measurable, achievable, relevant, and timebound (SMART) corrective actions to address the root causes.
- Assign responsibility: Assign responsibility for implementing each corrective action to specific individuals or teams. Timeline: Establish a timeline for the implementation of each corrective action
- Implementation and Monitoring: Implement the plan and evaluate its performance. Create mechanisms to gather data that can be used to measure the success of changes that were introduced.
- Implement changes: Put the corrective actions into practice. Ensure that staff are trained and aware of changes to procedures or policies. Monitor effectiveness: Continuously monitor the effectiveness of the corrective actions. This may involve regular audits, follow up assessments, and a feedback mechanism.
- Documentation and Reporting: Create documentation of the RCA process including all findings, root causes, and corrective actions taken. Share findings with stakeholders, promoting a culture of transparency.
- Detailed Reports: Document the entire RCA process, including findings, root causes, corrective actions, and implementation outcomes.
- Communicate findings: Share the RCA findings and action plans with all stakeholders including staff, patients, and regulatory bodies.
- Continuous improvement: Review the RCA process on a periodic basis to identify any necessary modifications or areas that could benefit from improvement. Continuous improvement ensures that the agency promotes a culture of growth and continuous learning.
- Review and revise: periodically review the RCA process and outcomes to ensure sustained improvements. Revise strategies as necessary based on new data and feedback.
- Promote a learning culture: Foster an environment where continuous learning and improvement are encouraged and staff feel empowered to report issues and participate in problem solving.
Root cause analysis is a vital tool in hospice care for understanding and addressing the underlying causes of adverse events and errors. By systematically identifying and correcting these root causes, hospice agencies can significantly enhance patient safety, improve quality of care, and promote a culture of continuous improvement. A well-executed RCA resolves the immediate issue and provides valuable insights to prevent future occurrences, thereby ensuring better outcomes for patients and their families.




0 Comments