
by editor | Aug 25, 2024 | Care Keys - Aides, Care Keys - Chaplains, Care Keys - Nurses, Care Keys - Social Workers, Rules and Regulations - Office Team, Rules and Regulations - Social Workers, Rules and Regulations - Volunteers
As a member of the hospice healthcare team, you play an important role in caring for your patients. Because of this, you will often learn private information about them – not just about their health, but also about their personal relationships, their financial situations, and other sensitive and personal information. It is important to understand that you have a legal and ethical responsibility to keep this information confidential and only share it – when necessary – with other healthcare professionals who are part of the patient’s care team. It is your responsibility to protect patient privacy.
Why is it important to keep healthcare information private?
In 1996, the Health Insurance Portability and Accountability Act (HIPAA) was passed to protect people’s health information. The main goal of HIPAA is to ensure that health information is kept private and secure, and only shared with those who need to know in order to provide care or process medical records. This law applies to everyone working in healthcare.
What does HIPAA protect?
HIPAA protects what is called “personal health information” (PHI). This includes any details that could identify a patient, such as:
- Name
- Medical record number
- Date of birth
- Address
- Email address
- Social security number
Only those directly involved in a patient’s care or those who handle billing or administrative tasks should have access to this information.
Your role as a member of the patient’s healthcare team
As a member of the patient’s healthcare team, it is important to follow HIPAA rules to protect your patient’s privacy. If you share a patient’s health information without permission, it can harm the patient and break the trust they have in you. Here are some important things to keep in mind:
- Do not share information unnecessarily: Never discuss a patient’s health with friends, family, or on social media. Only discuss patient care with other healthcare workers who are directly involved in that patient’s care.
- Keep conversations private: If you need to talk about a patient’s care with another healthcare worker, make sure you do so in a private place where others cannot overhear.
- Secure patient records: Whether you are handling paper records or using electronic systems, always ensure that patient information is stored securely.
Why following HIPAA is important
By following HIPAA regulations, you help protect your patient’s privacy, ensure their information is handled with respect, and build trust. Patients and their families rely on you to keep their personal information safe, and HIPAA provides the guidelines you need to do so.
What are the guidelines of not following HIPAA?
Hospices and their employees must protect patient information at all times. If HIPAA rules are not followed, it can lead to serious consequences including fines, penalties, and even imprisonment. This applies not just to the hospice itself but also to any vendors or contractors who work with patient information.
Final thoughts
Understanding and following HIPAA is an essential part of your job as a member of a patient’s healthcare team. By keeping patient information private, you help ensure their safety, comfort, and trust in the care they receive. Remember, protecting privacy is not just a legal requirement – it is a crucial part of providing compassionate and respectful care.
Where can you find more information
by editor | Mar 22, 2023 | Compliance and Regulatory - Directors, Metrics and KPIs, Rules and Regulations - Nurses, Rules and Regulations - Office Team
Patient length of stay is monitored to detect instances of inappropriate use of the hospice benefit or other deficiencies in the services delivered by the hospice provider. Length of stay is monitored for both very short length of stay as well as for length of stay that is longer than the norm.
What may unusual length of stay tell a hospice provider?
When patients are discharged alive with a short length of stay it may signal that the patient did not understand the hospice benefit when the patient was admitted to hospice. Or, patients may discharge live from hospice after just a few days because they were not satisfied with the services delivered by the hospice provider. Patients with length of stay longer than 180 days could be indicative of a patient who is no longer hospice eligible. Patients who are no longer eligible for service should be discharged from hospice and any payments that were received from Medicare while the patient was no longer eligible for services should be returned to Medicare. Failure to discharge the patient or failure to return the funds are examples of fraud and abuse.
How is length of stay calculated?
Length of stay is calculated based on the number of days that a patient receives hospice care. Specifically, for a patient who is discharged from hospice (whether or not the patient is discharged alive), the patient length of stay is calculated as follows:
Patient length of stay = [patient discharge date]-[patient admission date]+1
Which patients are included in length of stay calculation?
The length of stay calculation assumes that only discharged patients are considered in the calculation – since the formula expressly refers to the patient discharge date. When only discharged patients are considered (whether live discharges or discharges due to death), the hospice provider only has a backward-looking view on performance relating to length of stay. For example, if a hospice provider has been providing service to a patient for 12 months and the patient is still on service, the patient will not be included in the traditional average length of stay metric – since the patient has not yet been discharged. On the other hand, once the patient is discharged the patient’s length of stay will be at least 365 days since the patient – while still currently active – has already been on service for 365 days. If active patients are considered in a length of stay calculation, it gives a hospice provider a metric that can be used to highlight patients whose clinical charts and documentation of care may benefit from additional review.
What length of stay metrics should be calculated?
In addition to computing average and median length of stay based on discharged patients only, average and median length of stay can be computed for active patients. Patient length of stay for an active patient is calculated as follows:
Active patient length of stay = [end of evaluation period date]-[patient admission date]+1
For example, suppose the current date March 15, 2023 and a hospice wishes to calculate the active patient length of stay as of the end of 4Q 2022 for a patient who was admitted on December 1, 2022. The calculation is as follows:
- End of evaluation period date: 12/31/22
- Patient admission date: 12/1/22
- Active patient length of stay = (12/31/22) – (12/1/22) + 1 = 31 days
The active patient length of stay as of the end of 4Q 2022 is 31 days.
If the hospice wishes to calculate the active patient length of stay as of current date, the calculation is as follows:
- End of evaluation period date: 3/15/23
- Patient admission date: 12/1/22
- Active patient length of stay = (3/15/23) – (12/1/22) + 1 = 105 days
Average and median length of stay would be computed as usual. If any concerning value — such as long length of stay – is identified based upon the active patient length of stay, a hospice provider can immediately investigate and determine if any remediation action is required, rather than waiting until patients are discharged. Delay can lead to additional fines or further action from Medicare.
by editor | Mar 22, 2023 | Compliance and Regulatory - Directors, Metrics and KPIs, Rules and Regulations - Nurses, Rules and Regulations - Office Team
Patients are eligible for hospice if they have a terminal diagnosis and a prognosis of six or fewer months to live if their disease runs its natural course. A patient who lives longer than six months can still get hospice care if the medical director or other hospice physician recertifies that the patient is still terminally ill.
What is hospice patient length of stay?
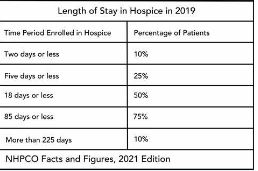
Hospice length of stay is an important metric that is monitored by both CMS and by hospice providers. Hospice length of stay measures the count of days that a patient receives hospice services, from the day that the patient is admitted into hospice until the day the patient is discharged (either alive or deceased). In 2018, 25% of Medicare beneficiaries received hospice care for seven days or less and 54% of Medicare beneficiaries received hospice care for 30 days or less.
Why should a hospice monitor patient length of stay?
Monitoring patient length of stay can aid in detecting cases of possible fraud or abuse – instances where ineligible patients continue to receive the hospice benefit. This metric also helps monitor whether the hospice benefit is being adequately utilized. Although patients are eligible for hospice when they have six months or less to live, most patients receive less than 30 days of hospice care.
Agency patient length of stay is also trended over time and is also compared against the value for patients in the same region, state, or nationwide. The metric may also be analyzed for patients in subpopulations – for example patients with the same disease, race, or ethnicity.
How is patient length of stay calculated?
Patient length of stay is calculated using all patients discharged by the hospice provider during the reporting period. For example, if the hospice would like to compute the length of stay for patients during the 4Q 2022, all patients who were discharged during 1Q 2023 would be included in the calculation. For each patient, the number of days from the date of patient admission until the date of patient discharge is counted; this represents the patient length of stay.
Patient length of stay = [patient discharge date]-[patient admission date]+1
What are common measures of length of stay?
Two common patient hospice length of stay measures are Average Length of Stay (ALOS) and Median Length of Stay (MLOS).
Average length of stay
Average length of stay is the arithmetic mean of the data collected. Specifically, if d is patient length of stay and N is the total number of patients then average length of stay (ALOS) is calculated as follows:
ALOS = ( d1 + d2 + d3 + …. + dn ) /N
Where di = patient length of stay for patient i
Median length of stay
Median length of stay is the middle number in the sequence of numbers. Specifically, compute the length of stay for all N patients. Then, order these N numbers in ascending order. The middle number is the median. If the number of patients is even then there is no middle number. Instead, the median is calculated by taking the average of the two numbers in the middle.
Comparing average and median length of stay
The average is sensitive to outliers in the data. That is, if there are a few patients with a very high length of stay while all other patients have a significantly lower length of stay, the average will be biased by these outliers and will give a misleading assessment of overall patient length of stay. Below, we give an example to provide greater intuition into the impact of outliers on average length of stay and the difference between mean and median length of stay.
Suppose a hospice agency discharged 35 patients during 4Q 2022. The patients’ lengths of stay are as follows:

We compute the average length of stay by summing each of the 35 patient’s length of stay (in the “Length of Stay” column) and dividing that total by 35 (the total count of patients).
Average length of stay (ALOS) = 38.5
We compute the median length of stay by sorting the patient’s length of stay in ascending order and identifying the central number. Since there is an odd number of patients, there will be a single central value. In this case, the central value is 20.
Median length of stay (MLOS) = 20
Average length of stay is almost double the median length of stay. What is leading to these significant differences between ALOS and MLOS? Observe the outliers in the data. There are two patients with length of stay that exceeds 200 days. There are two additional patients with length of stay exceeding 100 days. Since ALOS is sensitive to outliers, ALOS is being pulled to a higher value due to the presence of these outliers.
To provide additional insight, we have plotted a histogram of the length of stay values. A histogram shows the count of observations in the data that fall in each of the specified ranges.

The table on the left shows the count (frequency) of observations of patient length of stay in the data for each of the ranges: 0-10 days, 10-20 days, 20-30 days, 30-40 days, and greater than 40 days. There are 11 patients with length of stay between 0-10 days, 7 patients with length of stay between 10-20 days, 6 patients with length of stay between 20-30 days, 6 patients with length of stay between 30-40 days, and 4 patients with length of stay that exceeds 40 days.
Think about this histogram and now consider the MLOS and ALOS. Median length of stay is 20 days – it falls well in the middle of the data. Average length of stay, however, equals 38.5. It falls, essentially, in the final bar of this histogram and well beyond where the majority of the data lies. The provides a visual demonstration of the impact of outliers on ALOS.
Providers should monitor both ALOS and MLOS. Significant differences between these numbers would indicate the presence of outliers and should be investigated.
Print ‘n take hospice keys
- Understanding the difference between the average (mean) and the median
hospiceKeys-meanVsMedian
Where can you find out more?

by editor | Jan 29, 2023 | Billing, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Office Team, Hospice 101 - Social Workers, Intake, Rules and Regulations - Nurses, Rules and Regulations - Office Team
What is the False Claims Act?
The False Claims Act (FCA) was established in 1863 during the Civil War to combat fraud and abuse perpetrated by suppliers of the federal government. At that time, the law was referred to as “Lincoln’s Law.”
The FCA has evolved significantly in recent years and is now one of the main tools used by the government to fight fraud. The FCA penalizes individuals or entities that submit fraudulent claims to the government, cause fraudulent claims to be submitted, or conspire to submit fraudulent claims.
One of the noteworthy provisions of the FCA is the qui tam provision, also known as the whistleblower provision. The qui tam provision allows private citizens, also referred to as “relators”, to report details of alleged fraud to the government. The whistleblower “stands in the shoes” of the government to prosecute the claim. This action benefits the government and the taxpayer as well as potentially the relator, who may receive a share of what is recovered.
How does the FCA relate to a hospice agency?
The False Claims Act allows hospice agency employees, patients, families of patients, or any individuals with alleged knowledge of fraud or abuse by the agency to report the behavior. Under the qui tam provision of the FCA, the relator may be entitled to a percentage of recovered funds.
What are different types of false claims?
A claim is a request for money made to the government. A false claim is money that is obtained from the government due to false or fraudulent claims. False claims include claims where the service
- Has not been provided
- Is already included as part of a different claim (i.e., double billing)
- Is not coded correctly
- Is not supported by the patient’s medical record
Claims may also be false and are covered under the FCA if they result from a referral made in violation of the Federal Anti-kickback statue (Stark Law).
The False Claims Act also includes payment from the government based upon false certification.
False claims include claims that the hospice agency should have known were false or fraudulent.
What is a claim that a hospice agency “should have known” is false?
The FCA expressly includes claims that a hospice agency “should have known” were false or fraudulent. “Should have known” means deliberate ignorance or reckless disregard of truth. As such, a hospice agency cannot avoid liability by simply ignoring inaccuracy in their claims. Examples of “should have known” include:
- Ignorance of billing rules, i.e., lack of knowledge of the rules
- Failure to act on consistent trends that are indicative of inaccurate billing
- Failure to act on inaccuracies or system errors identified by outside or internal auditing teams
- Failure to correct inaccurate billing (impacting either past or future claims)
A hospice agency must understand the rules and take proactive measures — such as conducting internal audits within the organization — to ensure compliance and accurate billing.
How can False Claims Act matters be initiated?
There are two ways that FCA matters can be initiated:
- Initiated by the government: When a FCA matter is initiated by the government, this type of matter typically starts with an audit or an investigation by the government. The government would determine that there is a false claim made to it and would initiate a matter, usually by a subpoena or civil investigative demand (CID). The government would issue the CID directly to the hospice agency. CID is a form of subpoena that requires the hospice agency to engage in one-sided discovery. That is, the hospice agency is required to produce documents demanded, respond to interrogatories, and provide sworn oral testimony. However, the hospice agency may not conduct any discovery.
- Qui tam matter: this type of matter is initiated by a whistleblower, also known as a “relator,” typically through the filing of a sealed lawsuit in a federal district court. The hospice agency does not know about the qui tam lawsuit since the lawsuit is initially served on the government. The case remains under seal while it is investigated by the government.
What is the qui tam process?
Qui tam actions are initially filed under seal. That is, only the US Attorney and some members of the Department of Justice (DOJ) have knowledge of and access to documents related to the case. The relator serves the complaint on the government together with a written disclosure of all material evidence.
The purpose of the sealed qui tam action is to allow the DOJ time to evaluate the relator’s allegations and for the DOJ to decide whether it would like to take over primary responsibility for prosecuting the case. If the DOJ decides to take over primary responsibility for the case, the DOJ is said to “intervene.”
The complaint remains under seal for 60 days during which time the DOJ investigates the relator’s allegations. This 60-day period can be (and typically is) extended. In fact, the government may spend months – or even years – investigating the case.
While the DOJ conducts its investigation, it may issue a Civil Investigative Demand (CID). This form of subpoena requires the defendant (the hospice agency) to engage in one-sided discovery where the hospice agency must produce documents, respond to interrogatories, and provide sworn oral testimony, as demanded. The CID is “one-sided discovery” because the hospice agency may not conduct any discovery.
If the government decides to intervene, the government is then responsible for litigating the case and files its own complaint instead of the complaint that was filed by the relator. The relator remains a party to the complaint.
If the government declines to intervene, the relator may proceed in her own name subject to the government’s right to dismiss the claim or to intervene at a later date.
Whether or not the government decides to intervene, the government remains the real party of interest. (As a reminder, the relator is only “standing in the shoes” of the government.) As such, the government must agree to any decisions on the case. The relator may not agree to dismiss or settle the case without the government’s approval.
What are the key phases in a False Claims Act investigation?
- Phase 1: FCA investigation is triggered. Triggers may include:
- Qui tam (whistleblower) lawsuit
- Call to OIG hotline
- Information identified during audit or claim review
- Complaints
- Data mining
- Phase 2: Formal investigation launches. Investigation may involve:
- Review of corporate filings
- Interview current or former employees
- Review financial records
- Electronic surveillance
- Physical surveillance of employees or of company premises
- DOJ civil investigative demand (CID), or the like
- Government search warrant or raid
- Phase 3: Litigation or resolution
Who are common whistleblowers?
Anyone can be a whistleblower and anyone may report alleged fraudulent activity to the government. The most common relators are:
- Business partners
- Current or former employees
- Competitors
- Patients
- Individuals who mine CMS data to identify anomalies/FCA claims
How can a hospice agency reduce the chance of qui tam lawsuits?
Any complaints or concerns that are raised – by employees, vendors, patients, or competitors, or any other individuals should be investigated and treated with concern as these have the potential to reveal compliance issues that need to be resolved by the hospice agency.
Employee complaints – whether from departing or active employees – are often an excellent source of information on potential compliance issues. A hospice agency should have a clearly established method – that is clearly and often communicated to employees – for employees to raise concerns. It should also have an organized process to diligently investigate and address any concerns raised by employees.
- Internal complaints:
- There must be an organized process – that is communicated regularly to employees – for employees to raise concerns
- All concerns must be investigated
- Have a plan to address any issues that are identified
- Take any necessary corrective actions
- Follow up with the individual who raised the complaint
- Provide training, as needed
- Departing employees
- Treat employees fairly as they leave
- Conduct exit interviews to identify any potential compliance concerns – investigate any issues that may be identified
- Potential releases (e.g., recovery from FCA claims)
Employees must feel that there is a process for raising concerns and that their concerns are heard. Employees should not fear retaliation for raising concerns. A hospice agency should be diligent and careful to respond to all employee complaints that are raised internally or to any complaints that are raised when employees leave the organization.
What are the financial benefits of avoiding FCA violations?
False claims act matters can be quite costly for a hospice organization. In addition to returning the payments associated with the false claims identified and incurring the costs associated with attorney fees to defend the matter, the hospice agency potentially faces the following significant costs:
- Treble damages: The FCA has a treble damages provision which provides that a hospice agency that is found to have violated the FCA statute may be liable to pay three times the amount of the actual false claim amount
- Penalty per claim: Under the FCA, a civil penalty may be assessed for each false claim that is submitted. The civil penalty dollar amount per claim has increased with inflation and currently may be as much as $23,000 per claim.
Where can you find more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
All Medicare certified hospice agencies must submit an HIS Admission and HIS Discharge record on all admissions and discharges from their agency. The report must include all patients, irrespective of payer source, patient age, or location where hospice services were provided. It is recommended that data is submitted within 14 days to be sure that it is accepted within the required 30 day time frame. Submitting early will give the hospice agency time to adjust and correction the data, as needed.
What information is included in each HIS record?
- Admission HIS: Captured during the admission process
- Administrative information
- Preferences
- Active diagnoses
- Health conditions
- Medications
- Record administration
- Discharge HIS: sections of information captured during the discharge process
- Administrative information
- Service utilization (this has been replaced)
- Record administration
How are HIS records used?
The HIS record is used to compute seven process measures:
- Patient treatment preferences
- Beliefs/values address if desired by the patient
- Pain screening
- Pain assessment
- Dyspnea treatment
- Dyspnea screening
- Patients treated with an opioid who are given a bowel regimen
These process measures are combined to compute a single composite quality measure – the Comprehensive Assessment at Admission – that is reported on Care Compare. This composite measure assesses whether the seven key care processes were followed when a patient was admitted to hospice.
What are HIS Submission requirements?
- Within 30 days of patient admission or discharge of each hospice patient. All HIS records must be successfully accepted by QIES ASAP system within 30 calendar days of the patient admission or discharge date. See here for details on submitting HIS data
- – The requirements have included an incrementally increasing compliance threshold since data collection began. The Final Rule stated that beginning with FY 2018 reporting year, to avoid the 2 percentage point reduction in Annual Payment Update (APU), hospice agencies were required to submit at least 70% of their required HIS records within the 30 day deadline. For FY 2019 this minimum threshold was increased to 80% of all required HIS records. For FY 2020 and all subsequent years, the minimum threshold was increased to 90% of all required HIS records within the 30 day deadline. Hospice agencies that meet the submission threshold will avoid the 2% reduction in APU payment.
- – Non compliant providers, that is – providers that fail to meet this submission threshold, receive notification from CMS via a HQRP non-compliance letter that CMS sends via USPS and via the CASPER system. The CASPER letter identifies why the hospice agency is non-compliant and also provides information on how the hospice agency can request reconsideration. Agencies should monitor CASPER for receipt of such notice; agencies have 30 days from the date that the letter is sent for reconsideration.
How can a hospice agency validate that its HIS data has been accepted?
An agency can use reports in CASPER to monitor the status of HIS records submitted to QIES ASAP and track HIS record status, determine when correction of errors is required.
- The Hospice Timeliness Compliance Threshold Report enables a hospice agency to check the timeliness of acceptance of HIS records including the percentage of records that were submitted within the 30 day deadline to determine whether the agency will meet the required threshold.
- The Hospice Final Validation Report provides information on the status of submitted HIS files, indicated whether or not the records were accepted and details of any warning or error messages, if generated.
Where can you get more information?

by editor | Nov 27, 2022 | Compliance and Regulatory - Directors, Documentation - Chaplains, Documentation - Nurses, Hospice 101 - Aides, Hospice 101 - Chaplain, Hospice 101 - Nurses, Hospice 101 - Social Workers, Intake, Medical Records, Metrics and KPIs, Rules and Regulations - Chaplains, Rules and Regulations - Nurses, Rules and Regulations - Office Team, Rules and Regulations - Social Workers
The CAHPS survey is intended to measure the experience of patients who had died while receiving hospice care and the experience of their primary caregivers. It surveys informal caregivers – usually family members – of the persons who died under hospice care.
The survey is a component of the Hospice Quality Reporting Program (HQRP). It is an experience survey rather than a satisfaction survey. The intention of this survey is to provide data that can be publicly reported on Care Compare. It is also intended to provide hospice agencies with data for quality improvement.
How and when is the survey conducted?
To give the caregiver some time for recovery, the survey is administered to the primary informal caregiver of those who died while receiving hospice care at least two months following the month of the patient’s death.
The survey is conducted by mail, by telephone, or by mail with telephone follow up, at the hospice agency’s preference.
How are survey responses reported on Care Compare?
The survey is comprised of 47 questions. Not all of the respondents answer all of the questions and not all of the survey responses are publicly reported. Instead, some of the responses are aggregated together to generate a composite measure that is reported to the public. The result is that eight measures are publicly reported: six composite measures comprised of responses aggregated across multiple questions and two single item measures.
Composite measures:
- Communication with the family
- Receiving timely help
- Treating the patient with respect
- Emotional and spiritual support
- Help for pain and symptoms
- Training the family to care for the patient
Single item measures
- Ratings of the hospice
- Willingness to recommend the hospice
Which hospice agencies must participate in the CAHPS Survey?
Any Medicare certified hospice agency that served at least 50 survey eligible hospice patients in the previous calendar year and that received its CCN after January 1 of the previous calendar year is required to participate in the CAHPS Hospice Survey. The hospice agency is required to successfully submit 12 months of data, from January through December, of the data collection year. Failure to participate will result in a 2% penalty from Medicare payments.
Which agencies are exempt from participating in the survey?
- Newness Exemption: A hospice that receives its CCN on or after January 1 is eligible for a one-time exemption from the CAHPS survey for the remainder of that calendar year. For example, a hospice agency that receives its CCN in 2022 will be required to participate in the CAHPS survey beginning with patients who die in January 2023, unless the agency meets the Size Exemption
- Size Exemption: A hospice agency can apply for an exemption from the CAHPS survey if the agency served fewer than 50 survey eligible patients or caregivers in the prior calendar year. If multiple facilities share a single CCN, the survey eligible patients count is the total from all facilities that share the same CCN. The form to apply for the exemption, submission deadline, and further details on exemption, can be found here
Who administers the CAHPS Hospice Survey?
A hospice agency is not permitted to directly administer the CAHPS hospice survey. Instead, the agency is required to use a CMS approved survey vendor to administer the CAHPS surveys on an ongoing monthly basis.
Where are these results reported?
All eight CAHPS quality measures are publicly reported. They are all also available in the CASPER Preview Reports so that a hospice agency is able to review the data before it is publicly reported on Hospice Care Compare.
May a hospice communicate with its patients and their caregivers about the survey?
If a hospice agency wishes to let its patients know about the CAHPS survey, it must notify all patients about the survey rather than selectively notifying patients. Additionally, the agency cannot try to influence the survey responses or ask caregivers to give certain ratings.
How does CMS adjust the data that is submitted?
The data is case mixed adjusted. That is, CMS tries to remove the effects that arise from the demographics of the patients served by each hospice agency. The intent is to make the scores more comparable across hospice agencies. Data that a hospice agency may receive from its survey vendor may not be case mix adjusted. Consequently, the data that a hospice agency receives from its survey vendor may not match the data that it sees in the CASPER Preview Report or on Hospice Care Compare.
Can a hospice agency review its data before it is publicly reported?
CMS provides a 30 day review period during which providers can use the Hospice CAHPS Provider Preview Report to review their CAHPS data before it is publicly reported on Care Compare. This report can be accessed on CASPER. If a hospice agency finds an error in the data after review the Preview Report, it may request that CMS review the data by submitting a request to the following address hospicecaphssurvey@hsag.com. However, all requests for review must be submitted within 30 days of release of the Preview Report. Detailed instructions for requesting a review of the data can be found here
Where can you find more information?






